What is a Clinical Research Scientist?
If you've ever dreamed of being a detective but also had a knack for science, congratulations—you might be destined to become a Clinical Research Scientist (CRS)! These professionals don’t wear capes, but they battle diseases, pioneer medical advancements, and shape the future of healthcare. Want to know how they do it? Stick around, because we’re about to dive deep into one of the most rewarding careers in medical research!
Who is a Clinical Research Scientist?
A Clinical Research Scientist is a medical researcher who designs and conducts studies to improve healthcare. They investigate diseases, test drug effectiveness, and develop new medical technologies. Their work involves:
Studying disease patterns to develop better treatments
Ensuring the safety and efficacy of pharmaceuticals
Collaborating with medical teams to design clinical trials
Analyzing and interpreting complex medical data
Where Do Clinical Research Scientists Work?
CRSs work in a variety of settings, including:
✔️ Pharmaceutical companies – Developing and testing new medications
✔️ Biotechnology firms – Researching innovative treatments and therapies
✔️ Hospitals & universities – Conducting academic and clinical research
✔️ Government agencies (FDA, CDC, NIH) – Overseeing drug regulations and public health initiatives
Key Responsibilities of a Clinical Research Scientist
A CRS plays a multifaceted role in medical research. Their primary responsibilities include:
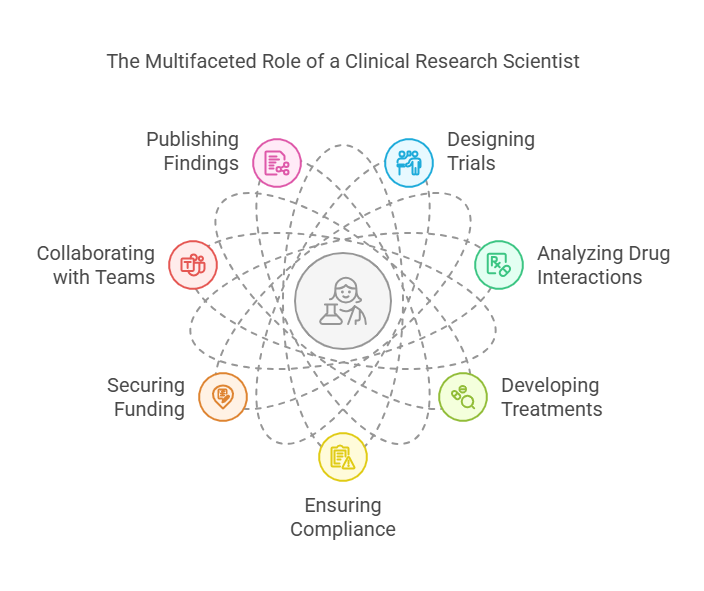
1. Designing & Conducting Clinical Trials
Clinical trials test new drugs, treatments, and medical devices. CRSs create trial protocols, recruit participants, and ensure adherence to regulatory guidelines.
2. Analyzing Drug Interactions & Side Effects
CRSs assess how drugs interact with the body and identify potential side effects. This ensures new medications are both safe and effective.
3. Developing New Medical Treatments
From cancer therapies to vaccines, CRSs contribute to groundbreaking medical discoveries that save lives.
4. Ensuring Compliance with Regulatory Guidelines
All research must follow strict regulations, such as FDA and ICH-GCP (Good Clinical Practice) guidelines. CRSs ensure studies meet these standards.
5. Applying for Grants & Securing Funding
Research requires funding, and CRSs write grant proposals to secure financial support from government agencies and private organizations.
6. Collaborating with Multidisciplinary Teams
CRSs work with doctors, statisticians, and regulatory experts to ensure research is accurate and ethical.
7. Publishing Research Findings
Findings from clinical research are published in medical journals to advance scientific knowledge and improve patient care.
Education & Certifications for Clinical Research Scientists
To become a CRS, you typically need:
Minimum Education Requirements
Master’s Degree – Most CRSs hold an MS in Biological Sciences, Pharmacology, or Medicine.
Ph.D. or MD – Higher education increases job prospects and earning potential.
Certifications for Career Advancement
Many professionals pursue certifications like:
Association of Clinical Research Professionals (ACRP) Certification
Society of Clinical Research Associates (SOCRA) Certification
ICH-GCP Training (For global clinical research compliance)
How Much Do Clinical Research Scientists Make?
According to the latest data:
Median Salary (2025): $98,750 per year
Top 10% Earn: $150,000+ per year
Entry-Level Salary: Around $65,000 per year
Job Growth & Demand
The demand for Clinical Research Scientists is increasing due to:
✔️ Growing medical advancements
✔️ Expanding clinical trials
✔️ Increased need for new drug approvals
Projected Job Growth: 9% increase from 2024-2034
Essential Skills for Clinical Research Scientists
To excel in this field, a CRS must have:
🔥 Strong Analytical & Problem-Solving Skills – Ability to interpret complex data
📊 Experience with Clinical Trial Software & Statistical Tools – Use of SAS, SPSS, and R for data analysis
🧠 Knowledge of FDA & ICH-GCP Regulations – Understanding of global research guidelines
📢 Excellent Communication & Teamwork Abilities – Collaborating with scientists, doctors, and regulators
If you’re serious about becoming a Clinical Research Scientist, getting proper training and certification is crucial. CCRPS (Certified Clinical Research Professional Society) offers industry-leading courses to help you master the skills needed for success in clinical research. Whether you’re a beginner or an experienced professional, CCRPS provides top-tier education that aligns with the latest industry standards.
Explore Courses for Clinical Research Career
Courses Available:
Frequently Asked Questions (FAQs)
-
A CRS designs studies, monitors clinical trials, analyzes results, and ensures regulatory compliance.
-
Not always! Some CRSs hold MDs, but many have Ph.Ds. in life sciences.
-
Rarely! They focus more on research, analysis, and trials.
-
Around 6-12 years, depending on education and experience.