How to Implement Effective Clinical Trial Management Solutions
CTM solutions play a crucial role in clinical trials by providing a framework for the management of clinical trials to ensure that each phase of the process is effective and compliant with regulatory requirements. The implementation of an effective CTM solution requires careful planning, the right tools, proper staff training, and integration with existing systems. This guide will provide you with a step-by-step approach to implementing a CTM solution, including how to choose the right tools, train your staff, and integrate the solution with your current infrastructure.
Step-by-Step Guide to Implementing a CTM Solution: From Planning to Execution
The implementation of a Clinical Trial Management solution follows several stages starting with the planning phase up to complete execution. The successful implementation of a CTM solution requires the following essential steps:
Define Your Objectives and Scope
Determine your goals: Establish your CTM solution objectives before starting the implementation process. Your CTM solution objectives include improving trial timelines and ensuring regulatory compliance and data management and stakeholder communication streamlining. Your decision-making process requires defined goals.
Scope of the project: Define the boundaries of your CTM implementation project. The scope of the project includes which trial phases (recruitment, monitoring, data collection, or analysis) need to be covered, budget, timelines, and available resources.
Conduct a Needs Assessment
Analyze existing systems: Review your current clinical trial management systems and processes. This will help identify gaps and areas where your CTM solution can provide the most value. Your team currently faces what challenges and pain points?
Stakeholder involvement: The planning phase should include early involvement of key stakeholders including clinical operations teams and IT departments and regulatory bodies to guarantee the solution will satisfy all needs and support organizational goals.
Select the Right CTM Solution
Evaluate available CTM tools: Review existing CTM solutions in the market and compare their features, scalability and compliance with regulatory standards such as GxP and FDA 21 CFR Part 11. Select a solution that can handle clinical trial data, documentation and workflows effectively.
Scalability and adaptability: Choose a solution that will grow with your clinical trials. You will need a CTM solution that can manage bigger trial volumes, more complex data and can be adapted to new regulations or methodologies.
Create an Implementation Plan
Set clear milestones: Create an implementation schedule with particular time points and target dates for each stage of the implementation process. This includes the software installation, configuration, testing, and full deployment.
Risk assessment: Identify potential risks or challenges that could delay the implementation process. Create contingency plans to address these risks as soon as possible.
Testing and Pilot Runs
Run pilot trials: Before implementing CTM across your organization, run pilot trials on a small scale. This helps identify issues with the software, workflow, or integration points and provides an opportunity to resolve them.
User feedback: During the pilot phase, collect feedback from users to know their experience, challenges and expectations.
Full-Scale Implementation and Execution
Deployment: After completing thorough testing of the CTM solution, move forward with its large-scale implementation. The deployment process should include all departments and teams and must have available resources for troubleshooting and support.
Monitor performance: Regular performance checks should be conducted on the CTM solution throughout its first few months of full implementation. Any problems should be resolved promptly while collecting data about system usage and efficiency.
Related Blog: The Future of Clinical Trial Management: Trends and Innovations
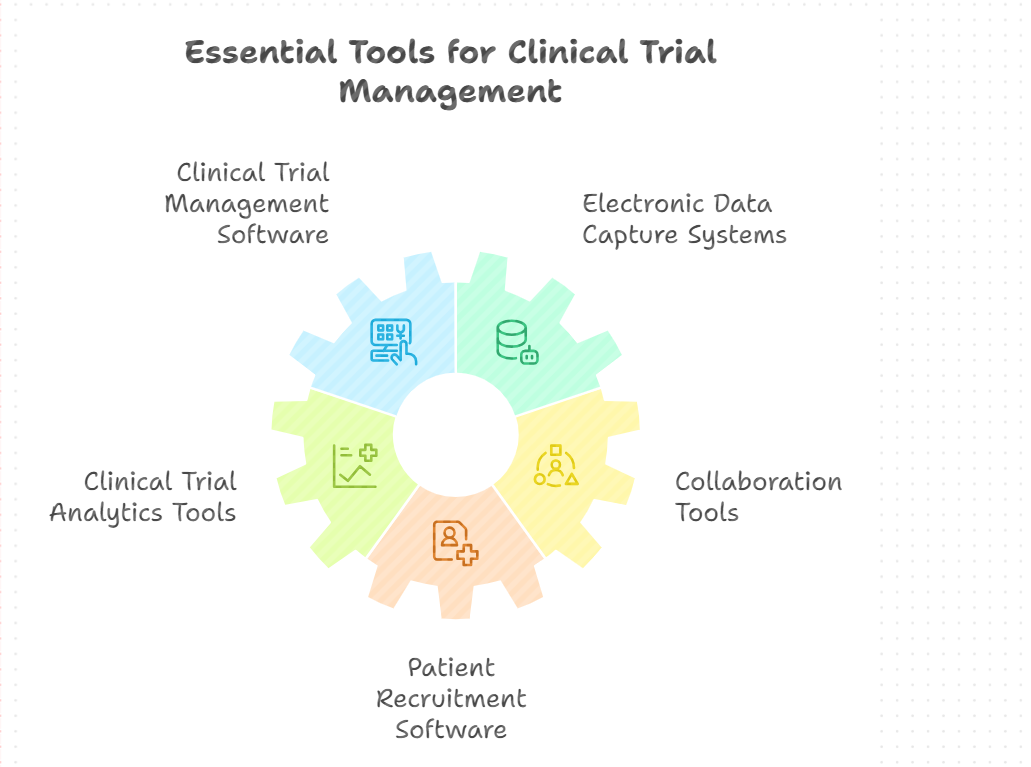
Selecting the Right Tools: Software and Technologies for CTM
The selection of appropriate software and technologies for your CTM solution determines its effectiveness. The following are the key tools and technologies to consider:
Clinical Trial Management Software (CTMS)
Comprehensive trial management: CTMS tools enable clinical trial management through patient data tracking and trial progress monitoring and study documentation management. The most commonly used tools for this purpose are Medidata, Veeva Vault QMS, and Oracle's Siebel CTMS.
Compliance and reporting: The software must adhere to worldwide regulatory standards and generate audit trails, document management, and reporting features to fulfill regulatory requirements.
Related Blog: How to Choose the Right Clinical Trial Management Software
Electronic Data Capture (EDC) Systems
Data collection: The electronic data capture (EDC) systems like Medrio or OpenClinica assist in the collection and management of trial data electronically. They provide real-time data access and minimize the risk of human error, thus leading to faster and more reliable data collection.
Clinical Trial Analytics Tools
Data analysis and insights: The tools deliver immediate analytics and real-time insights about the trial's progress and results. SAS and IBM Clinical Development and BIOSTAT provide detailed reporting tools which help optimize clinical trial operations.
Collaboration Tools
Team collaboration: CTM solutions can be integrated with software like Slack or Microsoft Teams for communication between clinical research teams, sites and stakeholders. These tools help in communication during trials in a timely and efficient manner.
Patient Recruitment Software
Recruitment and retention: The clinical trial process benefits from technologies including ClinOne and PatientNow which enable patient recruitment and tracking and retention throughout the trial. The platform enables fast patient enrollment and sustains participant involvement.
Training Staff: Ensuring That All Involved Parties Are Properly Trained
A successful CTM implementation requires proper training for all involved parties to learn how to use the new system. The following is how you can approach training.
Develop a Training Plan
Identify user groups: The level of training required will vary among different users including clinical trial managers, data entry personnel, investigators and IT staff based on their roles.
Create role-specific training modules: Create training modules which are specific to the roles and responsibilities of the users. Make sure that the system features that are relevant to each user group are covered so that they know the full capabilities of the CTM solution.
Hands-On Training and Simulation
Conduct live training sessions: Conduct hands-on training sessions which enable users to engage with the system while completing trial scenarios to learn about clinical trial management workflows.
Simulations and exercises: Conduct mock trials to enable users to gain hands-on experience with the CTM system. This will assist them in gaining confidence and familiarity with the software.
Continuous Education
Provide ongoing support: The organization should provide ongoing training and refresher courses to keep staff updated on system upgrades, new features, and industry best practices.
Knowledge base and FAQs: Build an internal knowledge base with useful articles, how-to guides, and FAQs to help staff after implementation.
Integration with Existing Systems: How to Make CTM Solutions Work with Current Infrastructure
CTM solution success depends on tool selection and staff training but also on how well the solution integrates with your current infrastructure. The following are steps to ensure smooth integration:
Assess Your Current Infrastructure
Review existing systems: Assess the current software systems and hardware infrastructure and established processes. Determine the data flow patterns that will connect your current systems to the new CTM solution.
Identify integration points: Identify the systems that need to be integrated with the CTM solution, such as electronic health records (EHR), inventory management systems, or patient databases.
Ensure Data Compatibility
Data migration: Develop a plan to move data from current systems into the new CTM solution. The data formats should be compatible and no critical information should be lost during the transfer.
API integrations: Your CTM solution should integrate APIs to establish connections with other systems including EDC and clinical trial databases. Real-time data sharing and platform updates will become possible through this integration.
Customizing the Integration
Tailor the solution: Tailor the CTM solution to the particular requirements of your clinical trials. This may include setting up workflows, user permissions and automated alerts in line with your current processes.
Test Integration Thoroughly
Pilot the integration: Before full deployment, test the integration of the CTM system with your existing infrastructure. Any issues should be identified and resolved during this phase to ensure that operations will run smoothly post-launch.
Monitor and Optimize
Continuous monitoring: After deployment, check the performance of the CTM system in conjunction with existing systems. Get feedback from users and stakeholders and improve workflows where necessary.
Related Blog: Best Practices for Clinical Trials and Data Management
10 Lesser-Known Facts About CTM:
CTM solutions are pivotal for global trials, ensuring compliance with diverse regulatory requirements across different regions. (Source)
Some CTM solutions support real-time data monitoring, allowing for proactive management of clinical trials. (Source)
Cloud-based CTM systems offer significant cost savings, especially for smaller research organizations with limited infrastructure. (Source)
Integration with EHRs ensures that patient data is securely transferred between clinical systems during trials. (Source)
Artificial intelligence (AI) is increasingly being used in CTM to predict patient enrollment patterns and optimize trial designs.
CTM systems can help mitigate the risks of protocol deviations, by automating trial workflows and ensuring timely data collection. (Source)
Some CTM tools offer predictive analytics, enabling trial managers to foresee potential delays and make necessary adjustments.
Mobile apps for CTM are gaining popularity as they allow trial managers and researchers to access real-time data on the go.
Blockchain technology is being explored for use in CTM to improve data integrity and security during clinical trials.
Certain CTM systems allow for remote monitoring of trial sites, reducing travel costs and improving efficiency.
Explore Courses for Clinical Research Career
Courses Available:
Conclusion
Implementing an effective Clinical Trial Management solution is a complex process that requires careful planning, the right tools, proper staff training, and integration with existing systems. By following a structured approach to selecting, training, and integrating CTM tools, organizations can enhance the efficiency of their clinical trials and ensure compliance with regulatory standards. With the right system in place, clinical trials can be managed more effectively, allowing researchers to focus on generating valuable insights that advance medical research.
For more information on CTM solutions and clinical trial management, visit CCRPS.
-
CTM involves managing the entire process of a clinical trial, including planning, execution, monitoring, and data analysis, ensuring compliance with regulatory standards.
-
Evaluate features such as scalability, compliance, data management capabilities, and integration with existing systems when selecting a CTM solution.
-
Key benefits include improved efficiency, better data management, enhanced regulatory compliance, and streamlined communication between stakeholders.
-
Training should be role-specific and may include hands-on sessions, simulations, and continuous education on system updates and best practices.
-
Integration involves assessing current systems, ensuring data compatibility, and using APIs to connect CTM software with other tools and platforms.