Clinical Trial Data Management Tools: What You Need to Know
New treatments require clinical trials to prove their safety and effectiveness while the rising number of worldwide trials has produced massive amounts of data. Data management efficiency has transitioned from being optional to becoming essential. The management of data errors leads to trial delays and expensive mistakes while unsafe treatments might be approved.
Data management tools help to achieve the smooth operation of trials. These tools help capture, store, manage, and analyze the vast amounts of data produced during a clinical trial. There are several types of data management tools available, each serving specific purposes, such as Electronic Data Capture (EDC) systems, Clinical Trial Management Systems (CTMS), and Data Analytics Platforms.
This blog provides an examination of these fundamental tools by analyzing their characteristics and evaluating the leading tools from 2025 while determining which tools work best for different trial requirements.
Related Blog: What is Clinical Trial Management? A Beginner's Guide
Overview of Popular Tools: EDC, CTMS, and Data Analytics Platforms
Electronic Data Capture (EDC)
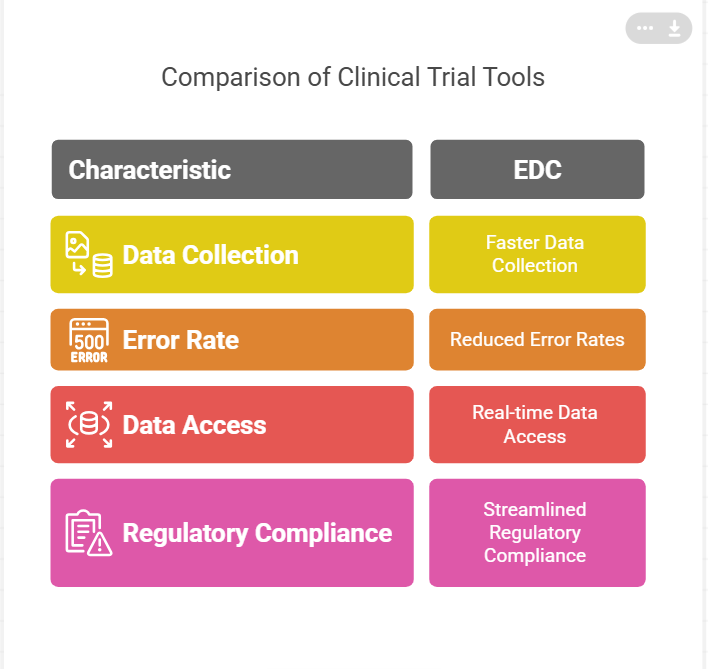
The main purpose of Electronic Data Capture (EDC) systems is to improve the efficiency of clinical trial data collection, storage and management. In the past, data was collected on paper forms, which was error-prone and time-consuming. EDC systems eliminate this problem by allowing data to be entered directly into an electronic system, which minimizes human error, speeds up the data entry process and provides real-time access to trial data.
The EDC systems allow clinical trial sites to enter patient data into electronic forms that are validated and checked for errors. These systems also help with monitoring data quality and ensuring compliance with regulations.
Key Benefits of EDC Tools:
Faster Data Collection: The immediate entry of data helps prevent delays which would happen if paper-based processes were used.
Reduced Error Rates: EDC tools assist in reducing the errors that are typical with manual data entry.
Real-time Data Access: Real-time data allows for faster decision-making and improved monitoring of trial progress.
Streamlined Regulatory Compliance: EDC systems usually have built in validation and audit features that assist with compliance with standards like FDA and EMA.
Popular EDC tools in 2025 include:
Medidata Rave: A widely used EDC platform known for its scalability and integration with other systems.
Veeva Vault EDC: The system provides an integrated, cloud-based system for managing trial data, documents, and workflows.
Oracle Clinical One: A unified platform for managing both EDC and CTMS functions, especially useful for large-scale clinical trials.
Clinical Trial Management System (CTMS)
Clinical Trial Management Systems (CTMS) operate to oversee all aspects of clinical trial management. The tools help with planning, tracking and managing different aspects of clinical trials including patient recruitment, site management, regulatory compliance and documentation. CTMS integrates and streamlines processes across departments, ensuring efficient collaboration and minimizing errors.
These systems enable clinical trial managers to track milestones, monitor patient enrollment, manage budgets, and communicate with other stakeholders involved in the trial.
Key Benefits of CTMS:
Comprehensive Trial Management: CTMS tools help in handling all aspects of a clinical trial, from patient recruitment to data management.
Enhanced Collaboration: These tools allow different teams to work together more effectively by centralizing trial information.
Streamlined Document Management: CTMS platforms often include features to manage study documents, including contracts, patient consent forms, and regulatory filings.
Real-Time Monitoring: Project managers and stakeholders can track real-time progress, which helps to prevent delays and identify potential issues early.
Some of the top CTMS tools available today include:
Veeva Vault QMS: Part of Veeva’s suite, this CTMS provides complete trial lifecycle management, including document management.
Parexel’s ClinPhone: A robust tool that integrates patient randomization, data collection, and monitoring into one platform.
Medidata CTMS: Known for its user-friendly interface and ability to scale for large trials, this CTMS integrates well with other clinical trial tools.
Related Blog: The Basics of Clinical Trial Data Management
Data Analytics Platforms
The importance of data analytics platforms in clinical trials has increased because data collection volumes have surged exponentially. The tools analyze clinical trial data to extract meaningful insights which enables researchers to base their trial decisions on data.
Data analytics platforms enable statistical analysis and data visualization and predictive modeling and machine learning to detect patterns and trends. They assist in optimizing trial design, assessing safety risks, and improving trial efficiency.
Key Benefits of Data Analytics Platforms:
Advanced Data Analysis: These platforms can analyze complex datasets and provide insights that would be difficult to identify manually.
Predictive Modeling: Data analytics tools use predictive algorithms to forecast trends and outcomes, which can significantly enhance decision-making.
In-Depth Data Visualization: These platforms offer intuitive visualizations of complex data, making it easier for teams to understand and act on the data.
Improved Decision-Making: With better analysis and forecasting, clinical trials are able to make more informed decisions, ultimately improving patient outcomes.
Some of the leading data analytics platforms in 2025 include:
SAS Analytics: A powerful analytics platform that has long been a staple in clinical trial data analysis.
IBM Watson Health: Known for its AI-driven analysis capabilities, this platform offers deep insights into clinical trial data.
Tableau for Clinical Trials: A visualization tool that helps researchers and trial managers create intuitive data dashboards.
Features to Look for in a Data Management Tool
The selection of a data management tool for a clinical trial requires multiple factors to guarantee the system will fulfill the unique requirements of your trial. The following features should guide your selection process:
Usability
A data management tool’s ease of use is critical. Trials often involve many stakeholders, including researchers, data managers, clinicians, and regulatory bodies. A tool that is intuitive and user-friendly will reduce training time, prevent mistakes, and allow trial teams to focus on what really matters: the patients and data. Users can interact with the system more efficiently through features which include customizable dashboards and drag-and-drop functionality and clear navigation.
Compliance
Clinical trials require absolute adherence to regulatory compliance. The data management tool needs to meet industry standards that include both Good Clinical Practice (GCP) and FDA 21 CFR Part 11 regulatory requirements for electronic records. The regulations maintain data accuracy and completeness while providing audit capabilities.
The system should have compliance features that include audit trails (a record of all changes made to data), automated validation rules, and robust access controls to protect sensitive information.
Data Integration
The research involves multiple sites and different data sources in clinical trials. A good data management tool should be able to integrate with other systems such as EDC, CTMS and laboratory systems. This minimizes the need for manual data entry and also ensures that all data sources are up to date. This feature is very important in order to ensure the quality of the trial data.
Security
Security stands as the primary factor to consider when selecting data management tools because clinical trial data remains highly confidential. The platform needs to implement strict data encryption standards while requiring multi-factor authentication for user access and maintaining detailed audit trails. Clinical trial data breaches create devastating consequences which endanger both trial integrity and all involved parties.
Scalability
The complexity of clinical trials tends to increase with time. You may need to scale up as the trial becomes multicenter or when you are dealing with large amounts of data. The data management tool you select should be able to handle this growth without performance issues. A scalable solution will save time and money in the long run by avoiding the need for a system overhaul.
Related Blog: The Future of Clinical Trial Management: Trends and Innovations
Comparison of Tools: Pros and Cons of Top Data Management Solutions
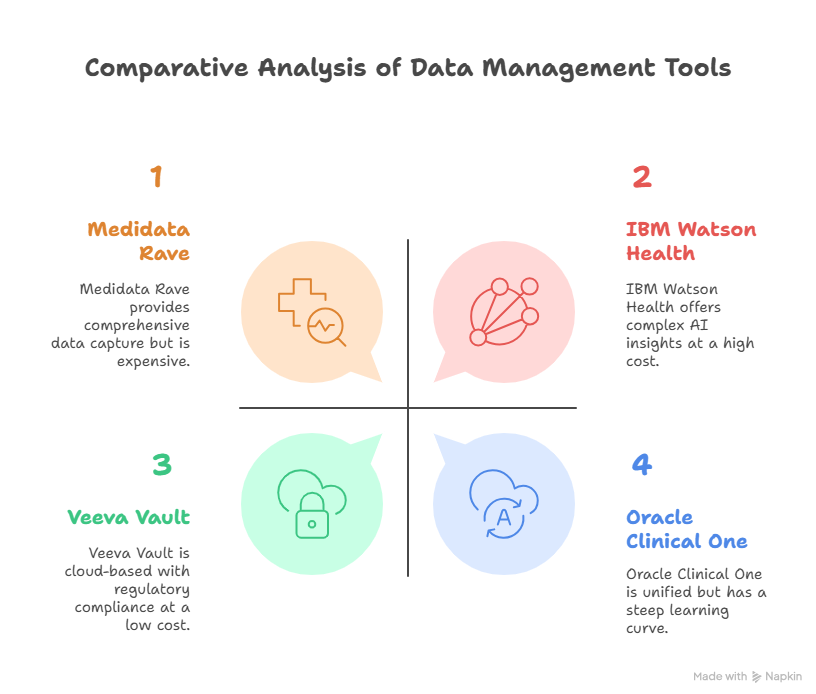
Medidata Rave (EDC Tool)
Pros:
Comprehensive Data Capture: Provides robust data collection and management features.
Seamless Integration: Easily integrates with other systems like CTMS and analytics platforms.
Real-Time Data Access: Enables users to access trial data instantly, ensuring faster decision-making.
Cons:
Cost: The platform is expensive, making it less accessible for small trials or research teams with limited budgets.
Complex Configuration: Some users report that the system can be challenging to configure for specific needs, particularly with custom trial designs.
Veeva Vault (CTMS and EDC Tool)
Pros:
Cloud-Based: This allows for real-time updates and access from anywhere.
Regulatory Compliance: Provides extensive compliance features, making it easier to adhere to industry standards.
Integration: Easily integrates with other tools, ensuring smooth data flow.
Cons:
Overwhelming for Small Teams: The complexity and breadth of the platform may be overkill for smaller trials or research teams.
Customization: Some users find it difficult to customize the platform to their exact needs, limiting flexibility.
Oracle Clinical One (EDC and CTMS Tool)
Pros:
Unified Platform: Offers both EDC and CTMS functionality in one system, reducing the need for multiple tools.
Regulatory Compliance: Strong emphasis on regulatory compliance ensures that trials meet all necessary legal and ethical requirements.
Scalability: Works well for large-scale clinical trials with many sites and complex data.
Cons:
Steep Learning Curve: Due to the complexity of the platform, there can be a steep learning curve for new users.
Cost: Oracle’s solutions are often considered expensive, especially for smaller organizations or trials.
SAS Analytics (Data Analytics Tool)
Pros:
Advanced Analytics: Offers powerful statistical and machine learning capabilities.
Big Data Handling: Excellent for analyzing large datasets and extracting meaningful insights.
Industry Trusted: SAS is widely used in the clinical trial industry, ensuring reliability.
Cons:
Learning Curve: Due to its complexity, SAS requires specialized training to use effectively.
Expensive: Licensing costs can be prohibitive for smaller trials or research teams.
IBM Watson Health (Data Analytics Tool)
Pros:
AI-Driven Insights: Uses AI and machine learning to uncover patterns and trends that would be difficult to identify manually.
Real-Time Data Processing: Watson processes data quickly, enabling faster decision-making.
Integration with EDC/CTMS: Can easily integrate with existing data management systems.
Cons:
High Licensing Fees: The platform’s cost may be prohibitive for smaller organizations.
Complexity: Requires specialized knowledge to fully leverage the platform’s advanced features.
10 Lesser-Known Facts About Clinical Trial Data Management Tools
Early Adoption of EDC: EDC systems emerged in the early 1990s, but it wasn’t until the 2010s that they became mainstream in clinical trials, largely due to advancements in cloud technology. (Source)
Mobile Compatibility: Modern EDC tools now offer mobile applications that allow data managers and clinical teams to access trial data and perform monitoring tasks from their smartphones or tablets. (Source)
Data Visualization Benefits: Data analytics tools are equipped with advanced visualization capabilities, which can turn raw trial data into graphs, charts, and dashboards, helping researchers make quicker and more accurate decisions. (Source)
Blockchain for Data Integrity: Blockchain technology is being tested to help improve the integrity of clinical trial data, making it tamper-proof and ensuring that records cannot be altered after they have been created. (Source)
Wearable Devices Integration: Many clinical trials are incorporating wearable devices to monitor patient health in real-time, with their data being integrated directly into clinical trial management systems for continuous analysis. (Source)
Global Data Standards: The adoption of global data standards, such as CDISC, is helping to streamline the sharing of clinical trial data between institutions and regulatory agencies.
AI-Powered Data Cleaning: AI is playing a pivotal role in clinical trials by automatically identifying and cleaning inaccurate data, helping to reduce human error and ensure data accuracy.
Real-Time Monitoring: Modern clinical trial management tools allow for real-time tracking of patient data and trial progress, which reduces the need for on-site monitoring and increases efficiency.
Data Security Advancements: Tools in clinical trials are increasingly adopting advanced encryption and access control mechanisms to safeguard sensitive patient information from cyber threats.
Automated Report Generation: Many data management tools now include automated reporting capabilities, which generate real-time reports based on the data collected, reducing the administrative burden on clinical teams.
Explore Courses for Clinical Research Career
Courses Available:
Conclusion
A clinical trial requires the appropriate data management tool to achieve success. The data management process benefits from EDC, CTMS and data analytics platforms which provide distinct features to achieve efficient data management. The appropriate solution enables data collection and storage and analysis which supports trial objectives and follows regulations while improving team collaboration.
As clinical trials continue to grow in complexity, investing in a reliable and scalable data management tool is essential for staying ahead in 2025. For those looking for the best in clinical trial data management, CCRPS offers cutting-edge solutions designed to meet the specific needs of your clinical trials, ensuring data integrity and operational efficiency.
-
EDC (Electronic Data Capture) is focused on the collection and storage of clinical trial data, while CTMS (Clinical Trial Management System) is geared towards managing the operational aspects of the clinical trial, such as patient recruitment and site management.
-
By automating tasks such as data collection, error checking, and report generation, these tools reduce administrative burdens, enhance collaboration, and ensure that all trial data is accurate and compliant.
-
Look for encryption, user access controls, multi-factor authentication, and audit trails to ensure that data is secure and protected from unauthorized access.
-
Yes, many data management tools allow you to integrate data from wearable devices, providing real-time insights into patient health and improving the trial’s data accuracy.
-
AI can automatically identify and correct errors, ensuring that the data is accurate and reducing the need for manual intervention, which can save time and prevent costly mistakes.