Medical Science Liaison Certification Exam Ultimate Study Guide
The Medical Science Liaison Certification is a strategic gateway for professionals aiming to lead scientific dialogue within the pharmaceutical, biotech, and medical device industries. Unlike generalized certifications, this credential validates your expertise in clinical data, scientific exchange, and KOL engagement—the three pillars of high-impact MSL work.
Employers are prioritizing certified candidates because they reduce onboarding time, elevate brand credibility in scientific discussions, and drive evidence-based alignment across commercial and medical affairs. Certification isn't about theory—it’s proof of field readiness in data-driven conversations, trial strategy, and regulatory fluency.
This guide delivers the exact study structure, tools, and focus areas required to pass the exam and transition into—or grow within—an elite MSL role. If you're targeting a career in medical affairs, clinical strategy, or therapeutic area leadership, certification is the foundation that turns qualifications into competitive advantage.
What is the Medical Science Liaison Certification?
The Medical Science Liaison Certification is a professional credential that validates your ability to function as a scientific and clinical expert in field-based medical roles. It’s tailored for professionals aiming to bridge the gap between pharmaceutical research and real-world clinical application. This certification proves you’re not just academically trained—you’re ready to influence therapeutic decisions, build trust with key opinion leaders (KOLs), and support compliant medical strategies.
Unlike generic healthcare certifications, this one is focused specifically on the MSL’s cross-functional role. That means everything from understanding pharmacovigilance data to interpreting phase III trial outcomes is tested. It's not a marketing badge—it’s a benchmark of your ability to translate scientific insights into strategic action.
The certification serves three distinct purposes:
Credentialing: It confirms you're trained in scientific exchange, evidence interpretation, and stakeholder engagement, key for high-stakes interactions with clinicians and researchers.
Standardization: It ensures consistency across MSL roles, especially in multinational teams that require alignment on clinical knowledge and compliance.
Career Mobility: It opens doors into medical affairs, clinical development, and regulatory communication—roles where certified expertise is increasingly non-negotiable.
If you're serious about stepping into the MSL space or leveling up within it, this certification isn't a nice-to-have—it's a strategic necessity.
Key Responsibilities of a Medical Science Liaison
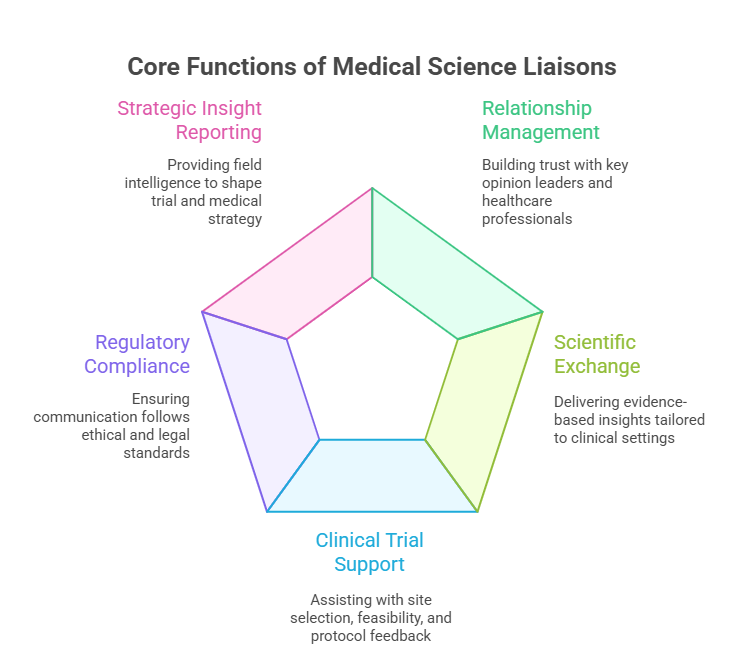
An effective MSL isn’t defined by a job title—it’s defined by impact. The Medical Science Liaison Certification equips professionals to operate at the intersection of clinical science, field engagement, and strategic execution. Below are the three pillars that define daily responsibilities in this high-stakes role.
Relationship Management with Healthcare Professionals
MSLs are the scientific face of their organizations. Their core responsibility is building evidence-based relationships with KOLs, physicians, and researchers. But it’s not sales—it’s clinical credibility. These relationships are grounded in trust, data integrity, and the MSL’s ability to facilitate accurate interpretation of clinical evidence.
This means:
Leading non-promotional discussions around trial results, adverse events, and MoAs
Identifying unmet needs and feeding that intel back into R&D pipelines
Maintaining consistent, compliant communication across therapeutic stakeholders
The ability to sustain these relationships is often what distinguishes entry-level MSLs from high-performing experts.
Scientific Exchange and Knowledge Sharing
A certified MSL must be equipped to deliver tailored scientific content that aligns with the healthcare professional’s needs. This isn't just about repeating trial data—it’s about interpreting evidence in context and adapting it for a specific clinical audience.
This involves:
Presenting abstracts and publications at medical congresses
Providing education on pharmacodynamics, safety profiles, and trial design rationale
Discussing off-label data compliantly under medical affairs governance
The Medical Science Liaison Certification ensures you're trained to walk this compliance tightrope while still adding scientific value.
Strategic and Tactical Support for Clinical Trials
MSLs play a pivotal role in clinical trial execution, even if they’re not managing the trials themselves. They’re often involved in:
Site identification and feasibility assessments
Supporting patient recruitment strategies via medical channels
Reporting back real-world insights from investigators to clinical teams
More than field reps, certified MSLs serve as a bridge between real-world evidence and protocol optimization. Their feedback can shape inclusion/exclusion criteria, inform endpoint feasibility, or uncover recruitment barriers.
Being certified means you’re equipped to provide field intelligence that matters—not just data, but insights that shift strategy.
Exam Structure and Requirements
The Medical Science Liaison Certification Exam is engineered to test more than memory. It evaluates whether you can interpret scientific data, apply regulatory standards, and communicate evidence in a compliant, high-impact manner. Understanding the format, requirements, and scoring model is non-negotiable if you’re aiming to pass on your first attempt.
Eligibility Criteria for the MSL Exam
To be eligible for the exam, you must demonstrate advanced scientific or healthcare background. Most candidates meet one or more of the following:
A degree in pharmacy, medicine, life sciences, or health sciences
Clinical research experience or scientific publications
Current or prior employment in medical affairs, clinical education, or scientific liaison roles
While a PhD or PharmD is not mandatory, it’s commonly seen among applicants. However, certification bodies prioritize field readiness and knowledge application over formal degrees alone. If you’ve worked in drug development, therapeutic education, or evidence-based outreach—even in adjacent roles—you’re likely a strong fit.
The certifying organization may also request a résumé, CV, or statement of interest, which should highlight your exposure to clinical science communication and stakeholder engagement.
Overview of Exam Content
The exam is structured around key knowledge domains critical to the MSL role. While weightings may vary, the core categories typically include:
Therapeutic Area Knowledge – Anatomy, pathology, disease mechanisms
Clinical Trials & Study Design – Phase I–IV protocols, inclusion/exclusion, endpoints
Medical Affairs and Compliance – Regulatory frameworks, off-label guidelines, ethics
Scientific Communication – Data presentation, slide deck development, medical writing
Stakeholder Interaction – KOL engagement, field reporting, cross-functional alignment
Expect questions that require more than just recall—you’ll need to interpret clinical trial abstracts, evaluate mechanisms of action, or determine the correct action in compliance-laden scenarios. The Medical Science Liaison Certification is built to validate real-world problem-solving, not textbook memorization.
Exam Format (Multiple Choice, Short Answer)
The exam consists of approximately 100–120 questions, divided across:
Multiple-Choice Questions (MCQs): These test core knowledge. Be prepared for questions with multiple technically correct answers, where you must select the most accurate or most compliant one.
Short Answer or Case-Based Scenarios: These assess data interpretation, strategy, and decision-making under medical affairs rules. You may need to analyze a trial result and write how you’d discuss it with a KOL.
Time Limit: You typically have 120–150 minutes, allowing just over one minute per question.
Passing scores vary but usually require 70% or higher. Results are either immediate or provided within 5–10 business days depending on the testing platform.
To succeed, you must treat this not as a quiz—but as a professional simulation. Each question is a litmus test for your ability to operate in scientific, regulatory, and interpersonal domains simultaneously.
Study Materials and Resources for the MSL Exam
Preparing for the Medical Science Liaison Certification requires more than traditional reading. You need targeted, high-yield resources that develop your scientific reasoning, regulatory fluency, and communication sharpness. Below are the tools top performers rely on—organized by effectiveness, not popularity.
Recommended Books and Guides
Books are foundational, but only when curated. Generic clinical texts won’t help. You need material built for medical affairs, scientific exchange, and trial strategy.
Top picks:
The Medical Science Liaison Career Guide – Focuses on field responsibilities, KOL strategy, and career context.
Fundamentals of Clinical Trials by Lawrence M. Friedman – Provides the structural backbone for understanding trial design, endpoints, and regulatory phases.
Certification-specific prep manuals – If your certifying body offers a proprietary guide, start there. These often contain sample questions and blueprints aligned with test priorities.
Avoid content-heavy pharma books not designed for MSL learning—they waste time and dilute focus. Prioritize exam-relevant material that mimics scenario-based application.
Online Courses and Practice Tests
Digital training platforms give you what books can’t: real-time feedback and pattern recognition.
Look for courses that include:
Video lectures from certified MSLs or medical affairs trainers
Downloadable decks on topics like MOA discussions, MSL compliance boundaries, and stakeholder management
End-of-module quizzes with rationales
Mock exams simulating actual format and pacing
These simulate field-to-exam transitions—a key differentiator between passive learners and test-day performers. Practice tests are where you identify knowledge gaps, adjust timing, and rehearse decision-making under pressure.
Top sources: CCRPS (if certified through them), Coursera for medical writing and regulatory science, or any certification provider’s official platform.
Study Groups and Forums
Learning in isolation can create blind spots. Study groups—especially ones led by experienced MSLs—help you:
Clarify regulatory gray zones through discussion
Role-play KOL questions and practice delivering data-driven answers
Get exposure to case scenarios you may never encounter solo
Engage in forums like:
LinkedIn MSL groups
Reddit’s r/clinicalresearch and r/pharmacy
Private prep groups from the certifying organization
When structured right, these forums become real-time insight hubs where tactics are shared, confusion is cleared, and test-day confidence builds.
Remember, your materials are only as good as how you use, test, and reinforce them. That’s what separates MSLs who pass from those who restart.
| Resource Type | Value Delivered |
|---|---|
| Certification-Specific Books | Align with MSL role expectations and the actual exam blueprint |
| Online Courses | Include video lectures, slide decks, and practice tests for real-time prep |
| Practice Exams | Build exam muscle memory with timed, scenario-based questions |
| Peer Forums | Offer insight into commonly misunderstood topics and exam trends |
| Study Groups | Reinforce learning through collaborative discussion and mock drills |
Key Topics to Focus on for the MSL Exam
The Medical Science Liaison Certification doesn’t reward generalists—it rewards candidates who master specific, high-impact knowledge areas. Your study time should be allocated based on what appears most in case-based and applied exam questions. Below are the three most essential domains to prioritize.
Medical Terminology and Concepts
You must be fluent in therapeutic area vocabulary, pathophysiology, and how treatments interact with disease mechanisms. This isn’t limited to memorization—it requires understanding how to translate these concepts in real-time conversations with HCPs.
Core focus areas:
Disease progression and pathology (e.g., oncology markers, inflammatory cascades)
Pharmacokinetics (PK) and pharmacodynamics (PD)
Mechanisms of action (MOA) of common therapies
Expect questions where you’re asked to match clinical effects to drug classes, explain complex MOAs in lay terms, or identify potential contraindications based on clinical history.
The goal: Speak clinical and scientific language fluently enough to be credible in front of KOLs and investigators.
Pharmaceutical Regulations and Ethics
MSLs must operate inside strict compliance walls—and the exam ensures you know exactly where those are. This section can be the make-or-break zone for first-time test takers.
Study in-depth:
FDA, EMA, and ICH guidelines
Off-label communication rules and how to respond to unsolicited requests
The Sunshine Act, medical writing disclaimers, and publication ethics
Expect scenario questions where you’ll need to choose the only compliant path in a gray situation. You’re not just being tested on knowledge—you’re being tested on your ability to navigate ethical conflict with regulatory precision.
The Medical Science Liaison Certification ensures you don’t just know compliance—you can live it in the field.
Clinical Research and Trial Methodology
The backbone of MSL effectiveness is understanding how trials are designed, executed, and interpreted. You must be able to break down protocol logic and endpoint hierarchies on the fly.
Prioritize:
Phases of clinical trials and their objectives
Inclusion/exclusion criteria rationales
Endpoint types: primary, secondary, exploratory
Trial designs: parallel, crossover, open-label, RCTs
You’ll encounter case questions requiring you to assess trial feasibility, critique a study’s methodology, or explain results to a non-scientific stakeholder.
This is where many stumble—don't just read trial phases. Know what they look like in practical settings, and how to speak about them with confidence.
| Topic Area | What to Focus On |
|---|---|
| Medical Terminology | Understand disease states, mechanisms of action, and PK/PD relationships |
| Regulations & Ethics | Master FDA, EMA, and ICH guidelines, plus off-label and compliance protocols |
| Clinical Trial Methodology | Focus on trial phases, designs, endpoints, and inclusion/exclusion criteria |
| Scientific Communication | Learn how to present data, build compliant slide decks, and respond to KOLs |
| Stakeholder Engagement | Develop relationship strategies with investigators and cross-functional teams |
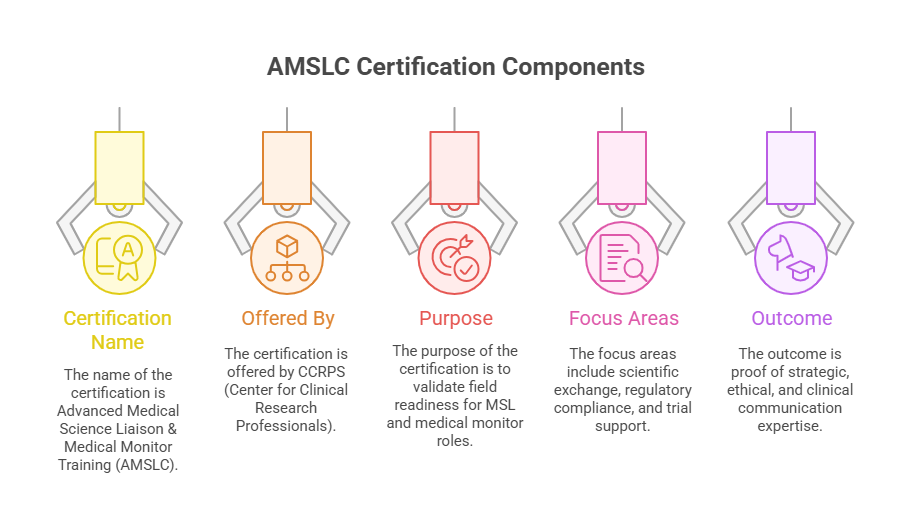
Why the AMSLC Certification by CCRPS is a Game-Changer for MSL Careers
Every strategy outlined above converges on a single outcome: qualifying and excelling through the Advanced Medical Science Liaison & Medical Monitor Training Certification (AMSLC) by CCRPS. This certification doesn’t just prepare you for a test—it prepares you to function as a strategic, compliant, and clinically credible MSL from day one.
AMSLC is designed specifically for professionals entering or transitioning into high-level MSL or medical monitoring roles. The curriculum is aligned with real-world trial oversight, scientific engagement, and regulatory frameworks MSLs face daily. That means your study efforts map directly to the core domains the certification validates—no wasted learning, no filler content.
Once certified, you gain:
Credibility with hiring managers and KOLs who expect field readiness, not just scientific theory
Eligibility for advanced MSL roles, medical monitor support positions, or global medical affairs teams
A credential recognized across pharma, biotech, and CROs as proof of medical affairs competency
Whether you're aiming to break into the industry or level up to senior field roles, AMSLC gives you the structured validation to back your experience—or fast-track your entry.
Frequently Asked Questions
-
To enroll in the AMSLC Certification by CCRPS, candidates typically hold a degree in pharmacy, life sciences, medicine, nursing, or a related clinical field. However, it’s not restricted to PhDs or MDs—many professionals with master’s-level education and relevant industry experience in clinical trials or medical affairs qualify. Prior roles in regulatory, clinical monitoring, or scientific communication further strengthen eligibility. While not mandatory, having experience with KOL engagement, trial protocol interpretation, or drug development enhances readiness. The program is designed to validate your ability to perform in a high-functioning MSL or medical monitor role—not just your academic credentials.
-
Most professionals complete the AMSLC Certification in 4–6 weeks with part-time study. The course is self-paced, allowing flexibility based on your schedule. On average, candidates dedicate 1–2 hours per day, focusing on modules covering scientific exchange, regulatory frameworks, clinical trial support, and ethical compliance. Fast-track learners can finish in under 3 weeks, while others take 8+ weeks depending on their familiarity with MSL responsibilities. What matters is mastery—not speed. The program emphasizes real-world application over passive reading, ensuring you're exam-ready and career-capable by the end.
-
The AMSLC exam is administered online and includes approximately 100–120 questions. It blends multiple-choice, scenario-based, and short-answer formats. Questions test your ability to interpret trial protocols, respond to off-label requests compliantly, and engage with KOLs using scientific accuracy and regulatory alignment. Most candidates complete the exam in 90–120 minutes. There’s no negative marking, and a passing score is usually 70% or above. Unlike typical knowledge-based exams, this one evaluates field-readiness and strategic judgment, so expect practical scenarios where choosing the most compliant and effective option is key.
-
The AMSLC Certification by CCRPS emphasizes key domains required for MSL and medical monitor success. These include clinical trial design and methodology, pharmaceutical regulations, KOL engagement strategies, scientific communication, and medical affairs compliance. Special focus is placed on the ability to interpret real-world evidence, respond to regulatory scenarios, and apply GCP-aligned decision-making in MSL contexts. You’ll also dive deep into stakeholder relationship management and how to support trial operations from a field medical lens. This is not just theoretical training—it’s structured to match the daily realities of advanced MSL roles.
-
Yes. While experience is valuable, the AMSLC Certification is structured specifically for those looking to transition into an MSL role or upgrade from adjacent fields like clinical research, medical writing, or regulatory affairs. The program provides both the technical knowledge and credibility needed to stand out to recruiters and hiring managers. By completing AMSLC, you demonstrate that you’ve mastered the language of medical affairs and understand the scientific, ethical, and compliance responsibilities that come with the role. It bridges the gap between theoretical qualifications and field expectations.
-
Yes, the AMSLC Certification by CCRPS is recognized globally by clinical research organizations (CROs), pharmaceutical companies, and medical device firms. CCRPS is known for offering high-impact, role-specific certifications that translate directly into career outcomes. Employers value AMSLC because it signals the candidate is trained, aligned with regulatory best practices, and capable of engaging in scientific exchange at a professional level. Unlike generalized programs, AMSLC aligns its curriculum with real-world job demands, making it highly relevant for hiring in MSL, medical monitor, and global medical affairs roles.
-
Earning the AMSLC Certification positions you for roles such as Medical Science Liaison, Medical Affairs Specialist, Medical Monitor, and Clinical Liaison across pharma, biotech, and CROs. It's also a launchpad for advancement into global medical strategy, therapeutic area leadership, and senior field medical positions. Certified professionals often move faster through promotion tracks because they arrive with both knowledge and regulatory-aligned communication training. AMSLC also enhances internal mobility if you're transitioning from roles like CRA, medical writing, or drug safety into field-based positions. It’s a career accelerator across medical functions.
Conclusion
Passing the Advanced Medical Science Liaison & Medical Monitor Training Certification (AMSLC) by CCRPS is not just about checking a credential—it’s about proving you're field-ready, compliant, and scientifically fluent. This guide has equipped you with the study strategies, focus areas, and resources that directly align with what the exam demands and what the role requires.
Whether you're breaking into your first MSL position or pivoting from a clinical or regulatory background, AMSLC gives you the strategic edge to rise above generic applicants. You now know what to study, how to retain it, and how to apply it when it counts.
Start executing. Eliminate guesswork. Elevate your career with structured mastery and credentialed proof.