Proven Test-Taking Strategies for Clinical Research Exams
You don’t pass a clinical research exam by just knowing the material — you pass by knowing how to handle the test environment like a systemized process. The difference between a pass and a retake often comes down to test-taking execution — not knowledge gaps. Most candidates preparing for the Clinical Research Certification by CCRPS fail because they waste time on low-yield methods, panic on uncertain items, or don’t simulate the real pressure they’ll face.
This guide isn’t about fluff tips or generic motivation. It’s a blueprint for test-day dominance — how to plan, perform, and recover with the same clinical discipline used in GCP-monitored trials. You’ll learn exactly what to expect, how to pre-load your mind with decision logic, and how to recover from curveballs in real time. Whether you’re sitting for your first clinical research exam or returning for a retake, these proven tactics are built to optimize your scoring, reduce cognitive fatigue, and align your performance with the reality of the test.
Know the Exam Format Before You Start Studying
Most clinical research candidates waste their first study weeks reviewing without understanding the exam’s mechanical structure. That’s like training for surgery without knowing what tools you’ll be handed. To prepare efficiently, you need absolute clarity on how the exam is built, timed, and phrased. The Clinical Research Certification by CCRPS is structured for precision — and demands test-takers who operate like strategists, not content hoarders.
Types of Questions to Expect
Expect application-based MCQs, not recall drills. These questions are designed to:
Present a scenario involving protocol deviation, informed consent, or adverse events
Ask for the most appropriate response, not just the “correct” one
Include multiple plausible options — but only one that is compliantly executable
You’ll also encounter:
“All of the following EXCEPT” logic traps
Questions that test GCP interpretation across global frameworks (FDA, EMA, ICH)
Decision trees involving SAE classification, PI responsibilities, and trial documentation
What this means: you won’t succeed by memorizing definitions. You need to build decision-making fluency under pressure, with compliance nuance baked into every answer.
Time Per Section Breakdown
You’re usually given between 90 to 120 minutes for 100–125 questions. That’s under 1 minute per question, with no buffer.
Plan like this:
First 30 questions: Move fast. These are usually warm-up or medium-difficulty. Average: 40–45 seconds each
Middle 50 questions: Likely the heaviest. Pacing must tighten — 35–40 seconds per question, using skip/flag for later return
Final 20–30 questions: Brain fatigue sets in. Use muscle memory and pre-planned tactics here — especially if time’s tight
No matter the section, you must respect the timer as your second exam. It’s not just about knowing — it’s about knowing on time. Practice accordingly.
Before the Exam: Setup Your Testing Strategy
Success in clinical research exams isn’t decided on test day — it’s locked in weeks earlier through systemized preparation. The candidates who perform well don’t just study hard — they train their brain to operate within the constraints of time, pressure, and decision fatigue. For the Clinical Research Certification by CCRPS, this means aligning your prep to the exact format, tempo, and role expectations the exam is built on.
Study Plan That Mirrors Exam Timing
Don’t block your study into static 2-hour cram sessions. Instead, design prep that mirrors exam pacing and duration:
Break your sessions into 50-question blocks with a 60-minute timer
Immediately post-block, review missed questions by category (GCP, consent, AE reporting)
Log root causes: misunderstanding, terminology trap, time pressure, or fatigue
Each week, increase difficulty: reduce time buffer, mix question complexity, and limit note access
This builds not just knowledge — it builds performance stamina.
Bonus: End every week with a realistic 100-question mock, simulating login, timing, and post-exam recovery. You’re not training for content — you’re training for execution.
Balanced Review vs. Rote Memorization
Memorizing isn’t learning. Most candidates fail because they over-commit to flashcards and neglect scenario fluency.
Here’s the better model:
60% scenario review (e.g., “What would you do if...?”)
20% compliance and GCP framework mapping
20% factual anchor review (definitions, timelines, reporting thresholds)
Why this matters: The exam rarely asks “What is an adverse event?” — it asks “Which of the following must be reported under GCP guidelines in this case?”
You need to know the rule — but more importantly, how and when to apply it under ambiguity. Focus on context-based understanding, not just term recall.
Practice Under Pressure Simulations
One of the most ignored test strategies: stress inoculation.
Build this into prep:
Weekly “crunch simulations”: 25 hardest questions, 20-minute limit, no review breaks
Use a countdown timer on screen, visible and ticking
Journal emotional spikes: When did you freeze? When did you rush?
Build response habits: breathe, skip, return, decide — no spirals
The more your body learns how to feel the stress and keep executing, the less anxiety impacts you on the real day.
During the Exam: Tactical Execution
Passing the Clinical Research Certification by CCRPS doesn’t depend on how much you remember — it depends on how consistently you execute under time pressure. Strategy isn’t optional. It’s what prevents fatigue, panic, and misjudgment from sabotaging your actual knowledge. You’ve prepped. Now you must perform like a technician: paced, tactical, and unshaken by traps.
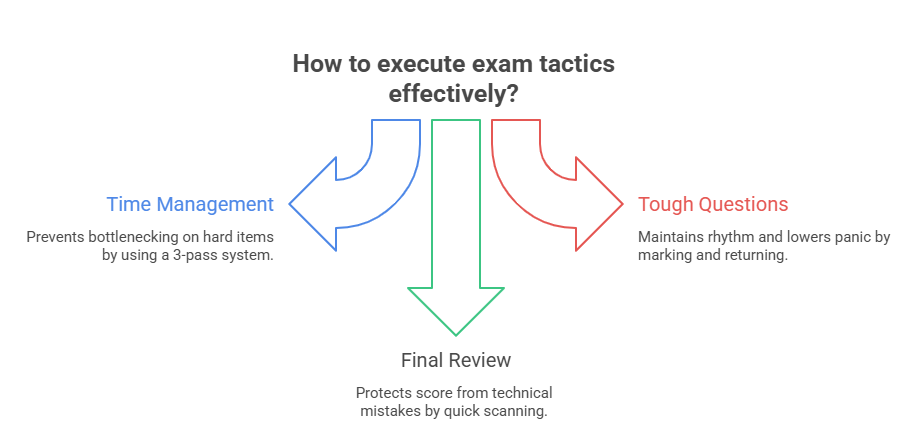
Managing Time and Skipping Wisely
Time is your invisible opponent. To win, you need a system — not vibes.
Here’s the model:
First pass: Answer only what’s clear within 40 seconds. Don’t debate.
Second pass: Revisit marked questions. Give them 60–90 seconds max.
Third pass: Quick review of all answers. Check for mismarks, but do not change unless 100% sure.
If you’re stuck:
Eliminate obvious wrongs
Choose best-fit based on GCP logic, not hunch
Flag and move
Never let a single question rob others of their time. One stubborn item can tank your entire score. Trust your process, not your perfectionism.
Mark-and-Return Technique for Tough Items
Tough questions are not the enemy — poor pacing is. Use mark-and-return to stay in control:
Mark any question that:
You’re 70% sure on but feel hesitant
Involves compliance gray areas
Has more than two plausible answers
Why it works:
Reduces cognitive overload
Prevents emotional stickiness (e.g., “I should know this!”)
Creates a recovery loop at the end when your brain is fully warmed up
By returning to tough items in the final 10–15 minutes, you engage them with greater clarity, reduced panic, and better intuition.
Bubble Management and Double Checks
Mis-clicks fail exams. You can’t afford it.
Final 5–10 minutes = buffer zone:
Scroll through to ensure every question has one selection
Look for two-bubble errors (rare, but fatal)
Do not change answers unless you misread the question entirely
Trust your first instincts — they’re built on prep and pattern recognition. The more you second-guess under pressure, the higher your error rate climbs.
Your final minute isn’t for fixing. It’s for protecting what you already got right.
After the Exam: Post-Processing Results
Most candidates either celebrate prematurely or spiral unnecessarily after submitting their exam. But if you want to grow — whether you pass or not — you need a clear post-exam process. What you do after the test matters almost as much as what you did before it. This is where high performers separate themselves: they reflect, recalibrate, and leverage the experience to build permanent exam fluency.
Reflection Review for Future Tests
Regardless of the outcome, do a same-day reflection review.
Capture:
What sections felt strongest and weakest?
Which question formats threw you off balance?
Did time ever become a threat — and when?
How well did your pre-planned strategies hold up?
Within 24 hours:
Write a post-exam play-by-play — from login to submission
Highlight emotional triggers: panic, confidence dips, or second-guess spirals
Rebuild your prep blueprint based on live data, not just score feedback
This creates cognitive closure, not emotional volatility. You don’t just survive the exam — you extract insight that makes future attempts stronger, shorter, and smarter.
Handling Unexpected Failures
If you didn’t pass, don’t patch it with optimism. Run a root cause debrief, not a guilt loop.
Ask:
Was it content familiarity, or decision logic under time?
Did anxiety spike in early questions or during the final stretch?
Were you guessing due to lack of prep — or panic?
Then, rebuild:
Narrow focus to question types and triggers, not entire domains
Practice on micro-timed quizzes — 10–15 questions at 30-second pace
Repeat simulations with scoring thresholds. Don’t stop at comfort — train to score under strain.
Failure is only failure if it goes unprocessed. With tactical post-exam review, it becomes data — not defeat.
| Post-Exam Step | What It Involves | Why It’s Critical |
|---|---|---|
| Reflection Review | Analyze pacing, weak domains, emotional triggers | Converts performance into actionable insight |
| Failure Handling | Identify root cause: content vs execution vs stress | Transforms setbacks into training data |
High-Yield Topics Worth Extra Focus
Not all exam topics carry equal weight. If you want to score higher with less effort, you need to target the clinical research topics that are most frequently tested — and most misunderstood. These areas appear consistently across question banks because they reflect real-world compliance pressure. For the Clinical Research Certification by CCRPS, these high-yield zones are where smart prep pays exponential returns.
GCP, ICH Guidelines
These aren’t just foundational — they’re woven into almost every exam scenario.
What to focus on:
Core ICH-GCP principles (E6 R2) — particularly subject rights, safety, and data integrity
Responsibilities of sponsors vs. investigators vs. monitors
Delegation of authority logs and essential document management
Differences in global regulatory expectations (FDA vs EMA vs PMDA)
Why they matter:
Many questions frame ethics as “what would you do if…”
Tricky distractors often exploit subtle wording in the guidelines
Scenario-based questions assume you know these frameworks cold
Don’t just read them — apply them in timed case-based questions until your response becomes instinctive.
SAE Reporting and Informed Consent
Expect 10–15% of your exam to center around safety events and participant protections.
Focus on:
AE vs SAE vs SUSAR classification — with exact thresholds
Reporting timelines (e.g., 24 hours, 7 days, 15 days)
Roles of site vs sponsor in event documentation
Re-consenting procedures after protocol amendments
Language requirements for consent forms under ICH and local regulations
Why this is high-stakes:
SAE mishandling is a compliance failure — the exam reflects this
Many distractors test whether you know who is responsible for each action
Questions often layer reporting logic with timing and ethics — you must think multidimensionally
Mastering these two topics means you can confidently tackle 30–40% of your exam without hesitation.
| Topic | Key Focus | Exam Relevance |
|---|---|---|
| GCP & ICH Guidelines | Principles, roles, essential documents | Forms foundation of 30–40% of questions |
| SAE Reporting | AE vs SAE vs SUSAR, timelines, responsibilities | Tests compliance fluency under ambiguity |
| Informed Consent | Re-consenting, language standards, protocol changes | Ethics-heavy, high-frequency scenario domain |
Why the CCRPS Clinical Research Certification Maximizes Exam Performance
Most certifications teach you the material. But the Clinical Research Certification by CCRPS goes further — it prepares you to perform under exam conditions. Its structure, delivery, and evaluation are all built with real-world exam psychology and professional execution in mind.
What sets it apart?
First, the CCRPS curriculum is scenario-driven, not fact-based. This mimics what you’ll actually encounter on the exam — complex, layered questions that test not just what you know, but how you think under pressure. Each module includes case applications, judgment challenges, and response mapping, so you’re not just passively consuming — you’re training your brain for decision agility.
Second, the program integrates compliance interpretation into every domain. You’re constantly exposed to ICH-GCP, SAE documentation flows, consent protocols, and global regulatory variance. This ensures that by the time you sit the exam, your instincts are already aligned with compliance-first thinking — exactly what certification scenarios are designed to test.
Third, the learning experience is modular and adaptive, which allows for realistic pacing. Candidates with full-time jobs or clinical roles can integrate prep into real schedules without burnout. More importantly, each section is followed by assessment drills that simulate question format, complexity, and timing, so you get immediate feedback on what to fix.
Frequently Asked Questions
-
Start by training with a timer in every study session. Set strict question pacing: 40–45 seconds per question, using a flag-and-return system for anything uncertain. Practice 100-question mock exams weekly under real time conditions — no breaks, no pausing. This builds mental pacing discipline. Use the three-pass strategy on exam day: (1) first pass for confident answers, (2) second for marked questions, (3) third for review only. Never get stuck on a single question — the exam is scored in bulk, not in perfection. Simulating time stress often is what allows your brain to stay focused, not flooded, during the real thing. Execution speed is a skill — not luck.
-
Clinical research exams prioritize scenario-based multiple-choice questions (MCQs), not straight memorization. Expect cases involving adverse event reporting, ICH-GCP protocol interpretation, ethics violations, and informed consent deviations. Many questions offer more than one plausible answer — only one will be compliant with both global guidelines and situational context. You’ll also encounter “All EXCEPT” and timeline-based reporting questions. The Clinical Research Certification by CCRPS emphasizes real-world execution, so you must apply frameworks — not definitions. If you’re preparing with only flashcards or notes, you’ll underperform. Use role-based case drills and live mock exams to build applied judgment.
-
Use the mark-and-return technique. If a question feels uncertain, select your best guess, mark it, and move on. Tough questions often come with overworded stems or overlapping answer choices — they’re designed to drain time. Don’t let one item sabotage your pacing. Once you reach the end of the test, return to flagged items with fresh eyes. Your brain will often resolve the ambiguity after processing easier questions, reducing panic. Prioritize questions where you can eliminate two choices confidently. Always choose the most compliant, risk-reducing action — not what feels intuitively “correct.” Consistency wins over correctness in high-stress conditions.
-
Yes — but only within a structured final review phase. Allocate the last 10–15 minutes to scan all responses for:
Incomplete or skipped answers
Double-marked or mis-clicked bubbles
Questions you flagged for return
Only change answers if you misread the question or discover a concrete reason (not a feeling). Avoid second-guessing unless you’re absolutely sure. Over-editing leads to score erosion. Your final review should act as a quality assurance pass, not a full re-take. This phase is especially important in digital exams with interface-based distractions. Review for technical errors first — logic errors second. Treat your final review like a clinical document audit — clean, quick, and deliberate.
-
Expect it. The worst thing you can do is be surprised by difficulty — or mistake it for failure. Start your exam with 3–5 warm-up questions to build rhythm. When you hit the first tough item, remind yourself: “Flag and go.” Early panic happens when candidates believe that difficulty = underpreparedness. That’s false. Clinical exams often shuffle question difficulty — one hard question doesn’t define your outcome. Breathe, stay on time, and return later with a fully activated mind. Pre-program a reset script: “I expected this. I trained for this. I move forward now.” Panic is not a command — it’s a cue.
-
Prioritize GCP, ICH guidelines, adverse event classification, informed consent, and regulatory timelines. These are the backbone of most question sets. Know the difference between AE, SAE, SUSAR — and their reporting deadlines. Understand sponsor, monitor, and investigator roles, especially in safety events and deviations. Questions love testing gray zones like document version control, re-consenting rules, and investigator delegation. Master protocol violations vs deviations, and when to escalate issues to IRBs or sponsors. These are high-yield and constantly repeated in various forms. Don’t just memorize terms — apply them in mock situations to build decision fluency under exam pacing.
Final Thoughts
The difference between passing and failing a clinical research exam rarely comes down to raw knowledge. It comes down to whether you trained for the environment, not just the material. Precision, pacing, and pattern recognition win — not overstudying or last-minute cramming. The Clinical Research Certification by CCRPS is structured to reward real-world thinking, not textbook regurgitation.
Every tactic shared in this guide is about regaining control when the clock is ticking, the pressure rises, and decision clarity matters most. If you’ve followed these strategies, you’ve already separated yourself from most candidates. You’re not just prepared — you’re strategically positioned to execute.
And that’s what this field rewards: execution under scrutiny, consistency under time, and judgment under ambiguity. Now go pass it like a professional.