Blogs
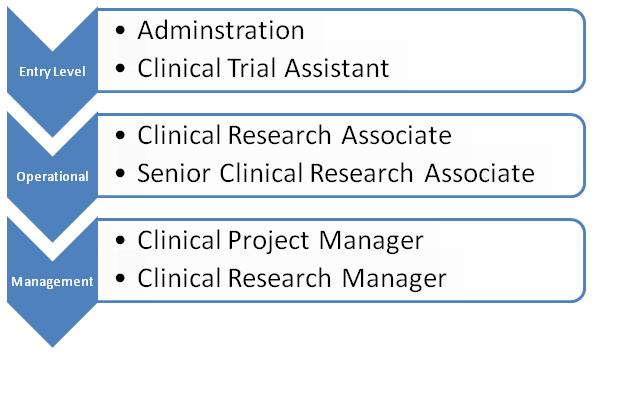
Clinical Research Project Manager

CRA Exam Questions
29.Allocable costA cost that can be assigned to a project or an activity based on the relative benefits received30.allowable only if approved by the sponsoring agencyAccording to OMB Circular A-21, costs incurred prior to the project start date are:31.All vertebrate animalsThe Federal Animal Welfare Act applies to which of the following animal species32.AnnuallyIf an institution of higher education is subject to OMB Circular A-133, when must it have an independent audit?33.The Anti-Kickback Act of 1986Which of the following aims at deterring subcontractors from making payments and contractors from accepting payments for the purpose of improperly obtaining or rewarding favorable treatment in connection with a prime contract or a subcontract relating to a prime contract?34.An anti-lobbying certification is required on federal grants, cooperative agreements and contracts exceeding a MINIMUM of:
a. $100,000
b. $500,000
c. $1,000,000
d. 10,000,000a. $100,00035.approval of student's embassyIn order for a foreign national doctoral student researcher to work on a federal grant in an American institution, it is necessary for the student to have36.Assure that the highest quality proposals are selected for funding in an equitable mannerWhich of the following is the primary purpose of federal proposal review processes?37.An award similar to a grant, and anticipates having substantial involvement in research activities once the award has been made.What is cooperative agreement?38.Award terms and conditionsA Notice of Award typically includes which of the following
39.Basic and containment procedures designed to protect personnel and the environmentWhich of the following best describes essential laboratory biosafety levels in medical research?40.Bayh-Dole ActWhich of the following was enacted as a uniform patent policy among the many federal agencies that fund research?41.BequestsA type of donation or gift with few or no conditions specified. Gifts may be provided to establish an endowment or to provide direct support for existing programs.42.Break refreshments at a project sponsored workshopUsing the criteria contained in 2 CFR 220, OMB Circular A-21, which of the following costs would most likely be allowable as a direct expenditure item on a federal grant?43.Budget period is yearly. Project period is the life of the contract.What's the difference between budget period and project period?44.a cabinet-level departmentThe Office for Human Research Protection is administered by45.A career development programAn employee in a position which does not enable realization of full potential would benefit most from46.cash flow statementAn important tool you may use to measure and track the flow of
cash into and out of your operation47.Certifications and representationsJust-In-Time initiatives postpone the submission of which of the following items until a decision to make an award is reached?48.Change in allocation within a single budget categoryWhich of the following changes does not require prior National Science Foundation approval?49.Change OrderA written order signed by the contracting officer, directing the contractor to make changes that the changes clause of the contract authorizes the contracting officer to order without the consent of the contractor.50.Chief Executive Officer or Vice President of ResearchWho appoints the chair for each committees at universities

Clinical Trial Monitoring Reports and How to Write Them
Among the aspects in study is observation. Overseeing the advancement of any stage, measure, process, and procedure in real time is essential to the accurate conclusion of any clinical trial undertaking. Normal monitoring actions are needed to guarantee caliber , efficiency, compliance with predefined and regulations fundamentals, in addition to comprehensiveness, and precision within clinical investigation. Such actions also ensure that the trial isn't just conducted in compliance with Standard Operating Procedures (SOPs) however they also function to validate it is correctly reported and recorded. There is something with a part in the execution of trials. And this thing is known as trial development reports.
Such a report ought to be carefully prepared and it must summarize the means that a study is done. It also ought to point out recruiting progress and procedures; should emphasize and clarify adjustments to the analysis, and ought to point security issues if there are not some.
Aside from the ethics committee, researchers could also be necessary to present yearly improvement reports of an investigation (such as any applicable alterations or dangers ) to spouses, encouraging associations, and/or organizations, along with other interested parties if needed.
If you're thinking about getting important skills on GCP or you also would like to upgrade your own know-how, subscribe to our comprehensive Great Clinical Practice class here.
In summary, tracking and reporting processes have an incredibly significant function in clinical trials. The right behaviour of these procedures not merely ensures compliance with legislation, regulations, and predetermined conditions, but in addition, it makes certain the study doesn't pose any dangers to their wellbeing. Progress reports, subsequently, empower practitioners, specialists, researchers, ethics committees, as well as others involved to keep a tab on the trial and its own advancement. They may signal any alterations or risks and may, therefore, react to them timely, correctly, and efficiently.
The objective of progress reports would be to accumulate and outline upgrades, key facets, along with summaries of a continuing trial. It's very crucial to be aware that progress reports must be filed to institutional evaluation board/independent integrity questionnaire (IRB/IEC), following a trial has obtained positive opinion.
One other important issue to mention is there are many different forms as soon as it comes to submitting progress reports that researchers must take into consideration before proceeding. Precisely, these kinds are:
Printing name and date of entry ought to be composed also. A digital copy is also needed to be delivered to the interested websites and committees inside a 30-day interval following the reporting procedure was completed.
The period of time whereby an advance report ought to be filed is at least one time in a year. Nevertheless, based upon the situation as well as the RECs' needs, these reports might be passed in more often, although the analysis is still going and till its official conclusion date.
2018 Clinical Research Associate (CRA) Salaries Estimated from 1,850 American Employees
Skills Alliance Clinical Research Associate
Clinical Research Associate Job available
$113,368 per year
Advanced Clinical Clinical Research Associate
Requires CCRP Online CRA Training Program Certification (Completed in 4 Weeks; you can apply 2 weeks into the course).
Clinical Research Associate Job available
$107,750 per year
Syneos Health Commercial Solutions Clinical Research Associate
Requires CCRP Online CRA Training Program Certification (Completed in 4 Weeks; you can apply 2 weeks into the course).
Clinical Research Associate Job available
$98,894 per year
Piper Companies Clinical Research Associate
Requires CCRP Online CRA Training Program Certification (Completed in 4 Weeks; you can apply 2 weeks into the course).
Clinical Research Associate Job available
$101,820 per year
Novella Clinical Clinical Research Associate
Requires CCRP Online CRA Training Program Certification (Completed in 4 Weeks; you can apply 2 weeks into the course).
Clinical Research Associate Job available
$97,659 per year
Covance Clinical Research Associate
Requires CCRP Online CRA Training Program Certification (Completed in 4 Weeks; you can apply 2 weeks into the course).
Clinical Research Associate Job available
$96,667 per year
Premier Research Clinical Research Associate
Requires CCRP Online CRA Training Program Certification (Completed in 4 Weeks; you can apply 2 weeks into the course).
Clinical Research Associate Job available
$95,178 per year
Syneos Health Clinical Clinical Research Associate
Requires CCRP Online CRA Training Program Certification (Completed in 4 Weeks; you can apply 2 weeks into the course).
Clinical Research Associate Job available
$93,469 per year
PRA Health Sciences Clinical Research Associate
Requires CCRP Online CRA Training Program Certification (Completed in 4 Weeks; you can apply 2 weeks into the course).
Clinical Research Associate Job available
$76,150 per year
Novo Nordisk, Inc. Clinical Research Associate
Requires CCRP Online CRA Training Program Certification (Completed in 4 Weeks; you can apply 2 weeks into the course).
Clinical Research Associate Job available
$95,242 per year
PPD Clinical Research Associate
Requires CCRP Online CRA Training Program Certification (Completed in 4 Weeks; you can apply 2 weeks into the course).
Clinical Research Associate Job available
$91,293 per year
IQVIA Clinical Research Associate
Requires CCRP Online CRA Training Program Certification (Completed in 4 Weeks; you can apply 2 weeks into the course).
Clinical Research Associate Job available
$89,615 per year
Cyberonics Clinical Research Associate
Requires CCRP Online CRA Training Program Certification (Completed in 4 Weeks; you can apply 2 weeks into the course).
Clinical Research Associate Job available
$87,000 per year
AbbVie Clinical Research Associate
Requires CCRP Online CRA Training Program Certification (Completed in 4 Weeks; you can apply 2 weeks into the course).
Clinical Research Associate Job available
$81,391 per year
ICON plc Clinical Research Associate
Requires CCRP Online CRA Training Program Certification (Completed in 4 Weeks; you can apply 2 weeks into the course).
Clinical Research Associate Job available
$79,110 per year
PAREXEL Clinical Research Associate
Requires CCRP Online CRA Training Program Certification (Completed in 4 Weeks; you can apply 2 weeks into the course).
Clinical Research Associate Job available
$81,281 per year
QuintilesIMS Clinical Research Associate
11 salaries
Clinical Research Associate Job available
$72,749 per year
MD Anderson Cancer Center Clinical Research Associate
7 salaries
Clinical Research Associate Job available
$71,410 per year
Chiltern International Clinical Research Associate
5 salaries
$87,722 per year

How to Prepare for a Clinical Research Interview
Preparing for any interview can be stressful, and clinical research interviews pose unique challenges that require specialized preparation. With advancements in the healthcare industry, competition is fierce, making it crucial to stand out.
How to Improve My AMCAS Work & Activities
Below are five simple ways you can improve your AMCAS Work & Activities
CCRPCOURSE.COM
Educate where you can
CCRPCOURSE.COM allows premeds in their gap year to be certified as Clinical Monitors to pursue a job where they have have expense-covered travel and oversee clinical trials run in medical schools around their region.
Expand your system
As a rigorous and open minded health care practitioner, you may be wondering exactly what you have to do in order to scale the corporate ladder quicker in the medical market. While the principle of'practice makes perfect' uses in the medical sector just like every other profession, progressing your health care profession will need more than your art in the business.
Remember that as you expand and grow, the exact same occurs for your health care career. The five projects highlighted below are the very first couple of actions that will assist you to get a location you would like from the area of medicine. Practice them, along with your healthcare profession will expand also.
Are your interests Dealing with your career route? Here is the very first question you need to have the ability to answer frankly in the event that you would like to improve your career in the health care market. Furthermore, if you're still not in the health care field, you need to ask yourself whether you may achieve the skills and instruction together with the experiences required to realize your objective. You might even elect for clinical research partner training for a means to improve your own comprehension.
Make the most of the onsite courses in addition to any instruction opportunities being provided. Besides that, additionally, there are hospital control and Clinical Research online classes being supplied at some prestigious schools, including McMaster University and James Lind Institute. As previously stated, choosing a clinical research partner instruction is a wonderful method to raise your probability of progressing since the longer you improve, the further you're generating opportunities for your own.
Stay ready, driven and motivated
You always should stay concentrated on the job at hand and being ready for everything and anything that comes your way. It is a very good method of demonstrating that you just take your career seriously. Regardless of what the job you're accountable for, whether large or little, always be ready for it. Additionally, don't forget to get centered on your potential career goals too, even though it feels like the progress you would like is farther away than you originally envisioned. Maintain those friends that are interested in exactly precisely the exact identical field near. You are able to think about these as motivators who drive you to keep on chasing your dream.
Picking a mentor has a few benefits, that will, in turn, assist you in improving your health care career. If you locate a suitable individual that's eager to take you under their wing, then begin with taking a good look at the job ethics and degree of knowledge. This will definitely point you in the ideal way, as you'll be asking yourself how you are able to be like these. Second, your mentor might assist you to network with other seasoned professionals who he/she understands.
Your present or former teacher from medical college will even share his wisdom and adventures with you. This may be a fantastic place to look for out information on whatever that you would like to learn regarding your health career. Utilizing the adventures of your mentor or boss since your aims may be the simplest route to follow if you would like to advance professionally.
Re-evaluate your livelihood
Another great approach to progress your career is by simply linking with likeminded people who work as caregivers. These professionals are available literally anywhere -- the establishment you've have your health care degree from, in your current (and past ) job, in seminars and conventions, in LinkedIn classes, etc.. ) If you would like to branch out farther than the men and women in your institution, you can attempt using online social networks to associate with more people just like you. Conventional social networks like working on your area and associations also work miracles.
