Clinical Research Trends For 2025
Picture this: You’re sitting at a coffee shop, sipping your overpriced latte, scrolling through headlines about AI-powered robots conducting clinical trials. Sounds like sci-fi, right? Well, welcome to 2025! The clinical research landscape is changing faster than you can say "randomized controlled trial," and if you blink, you might miss the next big innovation.
From AI and automation to decentralized trials and game-changing regulatory policies, this year promises groundbreaking advancements. So, buckle up, grab that coffee, and let’s dive into the top clinical research trends for 2025!
1. AI & Machine Learning: Revolutionizing Drug Discovery
Artificial Intelligence (AI) is no longer a futuristic fantasy—it’s a clinical research necessity. AI-driven algorithms are optimizing patient recruitment, predicting trial outcomes, and even analyzing massive datasets in real time. Companies like IBM Watson and DeepMind are leading the charge in AI-assisted drug discovery, significantly cutting research time.
Key AI Advancements:
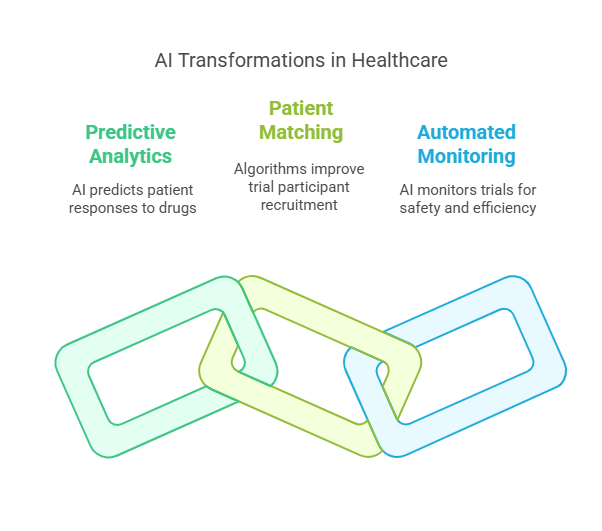
Predictive analytics for patient responses: AI can analyze genetic data, medical history, and lifestyle factors to predict how a patient will respond to a drug, reducing trial failures.
AI-driven patient matching for trials: Algorithms identify eligible participants quickly, improving recruitment efficiency and ensuring diverse representation.
Automated trial monitoring with real-time data processing: AI can detect anomalies, flag safety concerns, and optimize resource allocation in ongoing trials.
2. Decentralized Clinical Trials (DCTs) – The New Normal
With the pandemic accelerating virtual healthcare, decentralized clinical trials (DCTs) are now the gold standard. Instead of requiring participants to visit physical trial sites, DCTs leverage digital tools to conduct trials remotely. This approach improves accessibility and diversity in clinical research.
Key Features of DCTs:
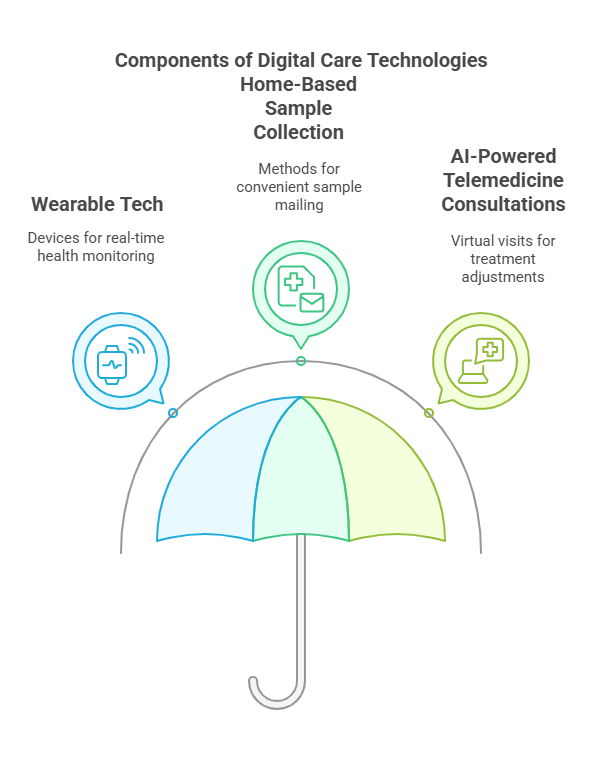
Wearable tech for remote monitoring: Devices like smartwatches and biosensors track patient vitals and medication adherence in real time.
Home-based sample collection: Patients can send blood, saliva, or urine samples via mail, reducing the need for in-person visits.
AI-powered telemedicine consultations: Virtual visits allow doctors to monitor patient progress and adjust treatments without physical interaction.
3. Real-World Evidence (RWE) & Big Data in Research
Forget small sample sizes—big data is taking over! Real-world evidence (RWE) is reshaping clinical decision-making by analyzing electronic health records (EHRs), insurance claims, and patient-reported outcomes.
How RWE is Changing Clinical Research:
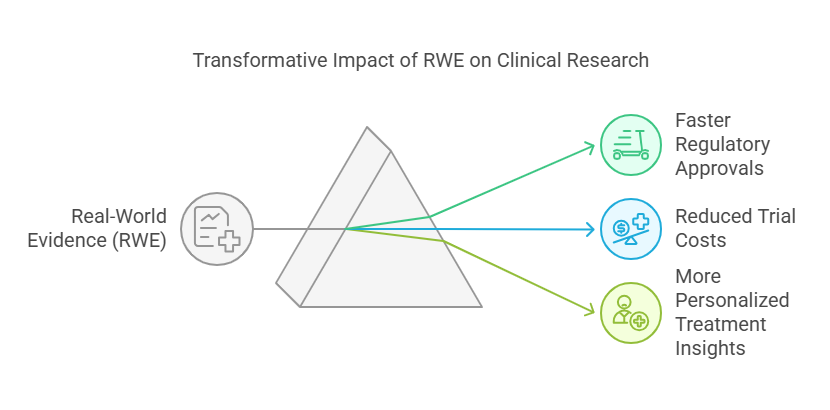
Faster regulatory approvals: Agencies use real-world data to evaluate treatment effectiveness before full-scale trials conclude.
Reduced trial costs: Using existing patient data eliminates the need for large-scale recruitment.
More personalized treatment insights: Researchers can tailor treatments based on real-world patient responses rather than controlled environments.
4. Gene & Cell Therapy Trials Expanding Rapidly
2025 marks a major leap in gene and cell therapy. Advances in CRISPR and CAR-T therapy are leading to personalized treatments for cancer, rare genetic disorders, and even chronic diseases like diabetes.
Breakthroughs in 2025:
FDA approvals for new gene therapies: More gene-editing treatments are receiving regulatory approval, bringing hope for previously untreatable conditions.
Expansion of cell-based immunotherapies: Personalized T-cell therapies are becoming mainstream for treating cancers and autoimmune diseases.
Use of AI to predict genetic treatment responses: AI models analyze patient genetic profiles to determine the most effective gene-editing strategies.
5. The Rise of Blockchain in Clinical Research
Blockchain isn’t just for crypto—it’s revolutionizing clinical trials! Secure, immutable records ensure data integrity, protect patient privacy, and improve transparency.
Why Blockchain Matters:
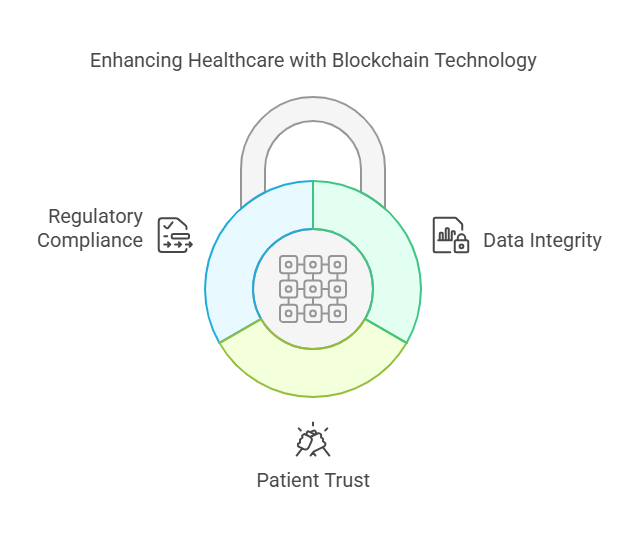
Prevents data tampering: Once recorded, trial data cannot be altered, ensuring reliability.
Enhances patient trust: Patients control their data and can track how it’s being used.
Enables seamless regulatory audits: Blockchain’s transparency simplifies compliance with regulatory bodies.
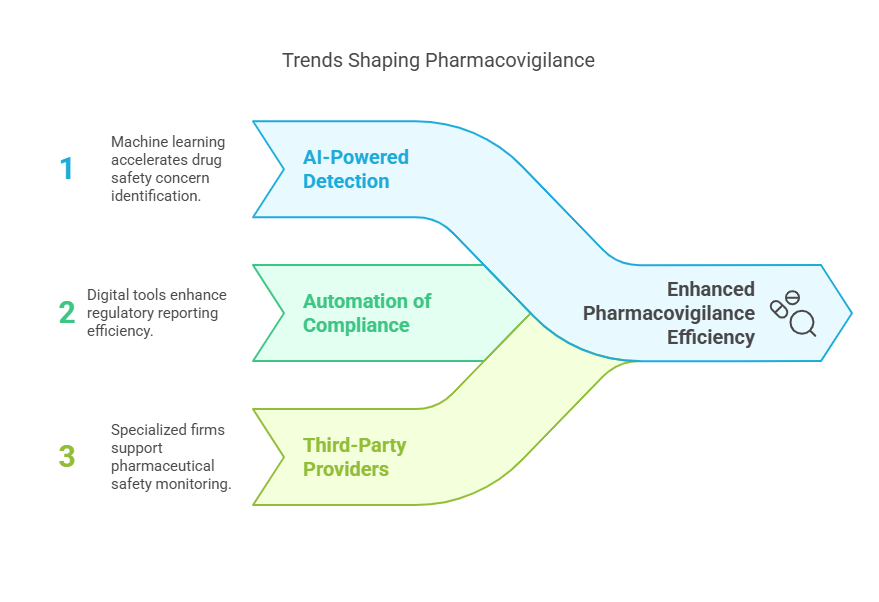
6. Pharmacovigilance Outsourcing: A New Era of Drug Safety
As regulations tighten, pharmacovigilance outsourcing (PVO) is on the rise. Companies are turning to specialized vendors for real-time adverse event tracking and safety reporting.
Trends in PVO:
AI-powered adverse event detection: Machine learning identifies potential drug safety concerns faster than traditional methods.
Automation of regulatory compliance processes: Digital tools streamline reporting to agencies like the FDA and EMA.
Increased demand for third-party pharmacovigilance providers: Pharmaceutical companies rely on specialized firms for safety monitoring and compliance.
7. Biotech Startups Going Solo
Big Pharma is no longer the gatekeeper of late-stage trials. Biotech startups are increasingly taking their own drugs to market, using Functional Service Provision (FSP) models to outsource key functions while maintaining control.
Why This Matters:
More competition in the industry: Smaller biotech firms challenge traditional pharmaceutical giants.
Faster innovation cycles: Independent biotech firms bring therapies to market quicker.
Greater focus on niche therapies: Startups target rare diseases and specialized conditions that big pharma often overlooks.
8. Regulatory Evolution: FDA & EMA's 2025 Guidelines
Regulatory agencies are embracing AI, real-world evidence, and patient-centric approaches. The FDA’s latest guidance promotes adaptive trial designs, while the EMA is emphasizing digital health integration.
New Regulatory Trends:
AI-assisted drug review processes: The FDA is using AI to speed up the approval of new treatments.
Standardization of real-world data usage: Agencies are setting guidelines on how to integrate RWE in trials.
Global harmonization of trial guidelines: Efforts are being made to unify trial regulations across different regions.
Conclusion
As clinical research continues to evolve, training and certification programs become even more essential. The Certified Clinical Research Professional Society (CCRPS) offers specialized programs to help professionals stay ahead of industry advancements. Whether you're looking to enhance your expertise in AI-driven trials, pharmacovigilance, or regulatory compliance, CCRPS provides the education and certification you need to thrive in 2025’s rapidly changing research landscape.
Explore Courses for Clinical Research Career
Courses Available: