Clinical vs Laboratory Research: A Comprehensive Comparative Analysis for 2025 – CCRPS
Clinical and laboratory research are both disciplines pivotal in the development of contemporary medicine, as each one integrates greatly into healthcare technology. Clinical research is the branch of medical sciences which focuses on evaluating new treatments for patients through human subject trials, while laboratory research centers around elucidation of scientific phenomena and its mechanisms through basic research. For 2025, technological improvements, regulatory changes, and shifting priorities in the healthcare sector are predicted to considerably impact both fields and their specialties.
Want to Work In Clinical Research Instead? Take 3 Question Career Assessment I View Certifications
The Evolution of Clinical and Laboratory Research
What is Laboratory Research?
Laboratory research is one of the types of research members of the academic community do. It is a scientifically controlled approach that seeks to understand biological, chemical, and physical phenomena in a defined environment. It encompasses performing experiments in defined specialized research facilities to analyze a particular set of theories, attempt novel medicinal cures, and appreciate the dynamics of a disease. Unlike clinical research, which has active human participants, laboratory research is essential for foundational breakthroughs that employ a cell culture, animal experimentation, or sophisticated computer modeling. This type of study is important to the success of developing new pharmaceuticals, medical equipment, and diagnostic agents before clinical trial laboratories perform human testing.
Advances in Clinical Trials and Research
Clinical research has advanced through greater focus on the patient and the application of modern technologies. There is a notable shift from conventional in-person examinations towards the use of decentralized clinical trials (DCTs). The introduction of wearable gadgets, comprehensive data collection through AI, and tele-health services has been set to enable the enhanced participation and cooperation of the medical scholarly community for broader and more efficient-claiming medical studies.
The integration of wearable technology, Real World Evidence, AI, and telemedicine solutions has made it possible for researchers to create more efficient, inclusive, and comprehensive medical studies. The clinical trial laboratories are key components in the health care system because they are responsible for a variety of activities such as collecting and processing biological samples and performing diagnostic tests to help with compliance. These laboratories serve as pivotal links in the chain between clinical research and regulatory sanctions, thereby maintaining the clinical research data quality.
Developments in Laboratory Research
These days laboratory research is exceedingly accurate and reliable. Applied research is when a study is done with the goal of solving marketable issues. Such developments include breakthroughs like high-throughput screening, the CRISPR gene editing, and advanced single-cell analyses. Fewer people will be skeptical about Simplifying cloud computing analytics and the IoT enabling AI significantly reduces the time between forming a medical hypothesis and the medical application of the hypothesis. New spheres of science and technology like synthetic biology and bio informatics are expanding the horizons of targeted scalable research even further.
Categories of Research Laboratories
Research laboratories are different according to their focus, methods used, and application in scientific and medical progress. Laboratories have been advancing in the incorporation of new technologies since 2025, and are now changing to improve precision and efficacy. Some 2025 types of research laboratories are:
Biomedical research laboratories: focus on human diseases and cellular structures to develop new medical treatments and therapies.
Pharmaceutical laboratories: spread in the processes of drugs discovery registration, formulation, and testing. These laboratories are critical when moving new medications from preclinical research to clinical trials.
Diagnostic laboratories: Analysis of disease detection and monitoring studied today, they work on analyzing patient samples, blood, urine tissue and other to aid healthcare decision making.
Biotechnology laboratories: These laboratories capitalize on biologic and genetic engineering for innovative and technology development in medicine, agriculture, and industrial goods.
Clinical trial laboratories: are specialists in assisting clinical research through biological materials sample processing, biomarker validation, and regulatory compliance monitoring.
Microbiology and pathology laboratories: Studied microorganisms and infectious agents and diseases pathology to assist in identifying resistance patterns.
All research laboratories work to achieve scientific advancement through understanding diseases, improving diagnostic methods, and developing therapeutic options prior to human clinical tests.
Table 1: Key Differences Between Clinical and Laboratory Research in 2025
Emerging Methodologies
Clinical Research in 2025
Advanced technology is making trials more efficient and accurate.
Patient Recruitment: Recruitment times have significantly increased as AI algorithms now scan vast datasets to identify eligible patients.
Data Collection: Participants’ health condition is constantly monitored through wearable devices.
Trial Models: There are now minimal barriers to participation due to the remote, decentralized nature of trials.
Stepped approach trials enable increased efficiency through making interim alterations to protocols in response to results.
Clinical Trial Labs: The Backbone of Research Validation.
In 2025 clinical trial automation, artificial intelligence (AI), and real-time data analytics are being used increasingly to increase productivity and reduce human error. Clinical trial labs are highly important as they determine the accuracy, compliance, reliability, and regulation of medical research. These labs convert biological materials into relevant forms for experimental use. They analyze biomarkers for verification and evaluate study results. Such functionality of clinical trial laboratories serves as a means for integrating research and practice, making them essential for all researchers in medicine.
Consider important activities of clinical trial labs:
Maintaining the Integrity of Biological Samples: Keeping and managing biological samples at a specific temperature to ensure their integrity throughout a study.
Biomarkers Discovery: Finding and validating disease specific biomarkers that will indicate the treatment outcome and response during clinical trials.
Good Clinical Practice Compliance: Compliance with GCLP and other regulatory bodies like FDA, EMA, and the ICH.
Laboratory Information Management Systems: Organizing and accommodating clinical lab data for efficient cooperation between scientists, pharma companies, and healthcare systems.
Clinical trial labs strive to increase safety and efficiency of the medical treatment process, hence these developments in automation and artificial intelligence enable faster and more accurate checkups of the research work done in the lab. In turn, medical treatments can be developed faster and additional research will be performed on new approaches to solving issues in the medical arena.
Laboratory Research in 2025
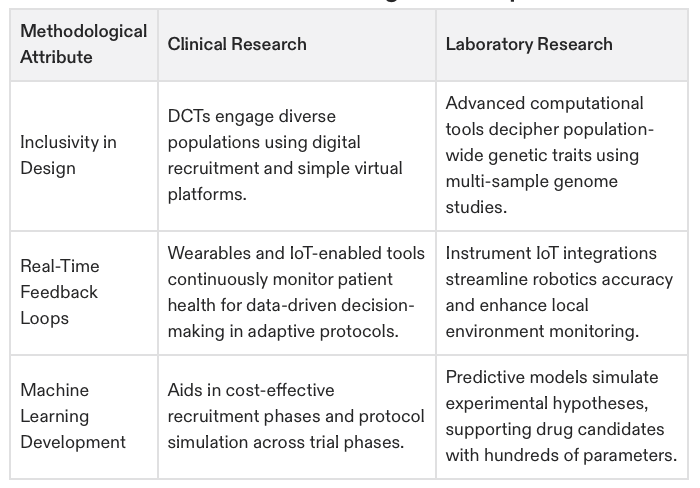
Laboratory research methodologies now balance advanced technology with methodological rigor.
Automation:
Robotic systems can simultaneously manage thousands of samples, enabling high-throughput workflows.
Advanced Modelling:
Computational tools powered by machine learning (ML) simulate outcomes with greater accuracy.
Multi-Omics Integration:
Combining genomics, proteomics, and metabolomics helps identify disease-specific biomarkers.
A structured approach to analyzing errors and conducting experiments can lead to improved methodologies that minimize errors and ensure systematic investigation in research processes.
Table 2: Methodological Comparison in 2025
Bridging the Gap through Collaboration
For 2025, increasing collaboration between clinical and laboratory researchers, where basic research is directly related to its applications in translational research, remains a primary force driving innovation. Enabling technologies such as cloud-based data systems, AI platforms, and cross-disciplinary training are breaking barriers between these two fields.
Key Collaboration Challenges
Data Integration:
Clinical data is often structured and dependent on human variability, while laboratory data is highly controlled. Bridging these requires advanced harmonization.
Communication Barriers:
Misaligned terminologies can hinder interdisciplinary teams from leveraging collaborative potential.
Solutions for Collaboration in 2025
Unified Data Platforms facilitate seamless data sharing between labs and clinical researchers, ensuring both teams work from compatible datasets.
AI-Driven Translational Pathways map molecular discoveries to real-world therapeutic applications.
Cross-Functional Training builds understanding between laboratory and clinical researchers, fostering effective teamwork.
Innovative solutions, such as digital workflows, can enhance the efficiency and continuity of clinical and laboratory research.
List 3: Collaborative Strategies in 2025
Unified cloud-based data sharing systems
Enables real-time insights, reducing research delays.
Regular interdisciplinary summits and workshops
Encourages knowledge sharing and alignment of research objectives.
AI-bridged workflows
Links laboratory-generated discoveries directly with clinical trials for faster validation.
Cross-trained roles (e.g., research hybrid positions)
Reduces operational bottlenecks through mutual comprehension of workflows.
Future Trends and Scientific Discovery for 2025
Clinical Research Trends
Hyper-Personalization in Medicine:
Greater emphasis on individualized treatments using real-world evidence data.
Decentralized and Hybrid Trials Expansion:
Combining traditional trial setups with remote elements ensures flexibility for global participation.
Regulatory Evolution:
Enhanced FDA/EMA oversight of adaptive clinical trials and expanded consideration of AI-generated insights.
Laboratory Research Trends
Synthetic Biology Boom:
Designing biological systems for novel diagnostic or therapeutic solutions.
Microfluidics Platforms:
Miniaturizing experiments for rapid assay processing and cost efficiency.
AI-Driven Laboratories:
Complete automation of repetitive tasks allows researchers to focus on innovation. Scientists play a vital role in interdisciplinary research teams, contributing significantly to advancing knowledge in laboratory research.
List: Predicted Trends for 2025
AI Integration
Predict recruitment challenges, reduce dropouts, and optimize trial protocols.
Enable predictive simulations for molecular discovery.
Remote Monitoring
Increased use of wearables for real-time patient health tracking.
Real-time integration of IoT devices for instrument feedback automation.
Personalized Medicine Enhancements
Tailoring therapies based on real-time multi-level participant data.
Rapid identification of biomarkers for stratified treatments.
Advanced Automation Systems
Improved reporting speed; fewer manual errors across recorded outcomes.
Reduced operational costs and faster adaptation to experimental demands.
FAQs
What is the main difference between clinical and laboratory research?
Clinical research involves testing interventions directly on humans, while laboratory research focuses on foundational experiments in controlled environments without human participation.
How do technological advancements affect these fields?
Technologies like AI, automation, and wearable devices have bridged gaps, improving efficiency and accelerating discoveries across both domains.
Why is collaboration between clinical and laboratory research important?
Collaboration ensures faster translation of discoveries into treatments, leveraging laboratory insights to guide clinical applications.
What are some future challenges foreseen for 2025?
Data harmonization across platforms, ethical considerations in AI-driven studies, and training interdisciplinary teams remain key challenges.