Research Assistant Certification Exam Comprehensive Study Guide
Breaking into the research field requires more than just academic curiosity—it demands proof of competence. The Research Assistant Certification offers that proof, validating your skills in research design, data handling, and ethical standards. Whether you're entering clinical, academic, or lab-based research, this certification is a strategic step forward in gaining employer trust, improving your resume, and accessing higher-salaried roles in regulated environments.
In this guide, we’ll walk you through everything you need to pass the Research Assistant Certification Exam—without the fluff. You’ll get clarity on the exam's content, the key subjects you need to master, and a breakdown of effective study strategies that work. We’ll also cover critical mistakes to avoid, how the certification connects to long-term career growth, and the most comprehensive answers to FAQs that usually trip candidates up. This isn’t just a guide—it’s your direct line to certification confidence.
What is the Research Assistant Certification?
The Research Assistant Certification is a formal credential designed to validate foundational research competencies. It certifies that a candidate understands core methodologies, adheres to ethical standards, and can support complex projects across academic, clinical, or industrial settings. Unlike traditional degrees, this certification offers practical validation of skills, appealing to hiring managers who need assurance that candidates can execute key tasks reliably.
Whether you're fresh out of school or transitioning into research from another role, this certification sends a clear signal to employers—you’re ready to contribute meaningfully from day one. Many labs, universities, and healthcare research institutions now list certifications like this as a preferred or required qualification, especially when handling sensitive data or assisting in compliance-driven projects.
The curriculum typically includes modules on research design, data collection, statistical principles, regulatory compliance, and subject safety. These aren’t taught casually—they’re structured to meet industry standards. Most programs, including the Research Assistant Certification from CCRPS, align their content with FDA and ICH-GCP guidelines. That means passing the exam isn’t just about memorization—it’s about mastering the real-world workflows of research professionals.
Below are two critical reasons this certification is a career asset:
Scope of Research Assistant Responsibilities
Certified research assistants are expected to take on responsibilities that extend far beyond clerical work. They assist in protocol development, manage research data, help with regulatory documentation, and even support ethical review submissions. In clinical research, they may also monitor subject interactions or manage informed consent records. This hands-on involvement makes their role vital to the success of every project.
A certification helps formalize this expectation. It ensures candidates know how to operate within legal and scientific frameworks, minimizing the risk of compliance violations or data mishandling. The broader your skill set, the more likely you’ll be trusted with higher-responsibility tasks—and that’s where the upward career path begins.
Benefits of Certification for Career Advancement
The certification acts as both a career accelerator and a differentiator. In job markets saturated with biology or psychology graduates, a Research Assistant Certification immediately sets you apart. Hiring Clinical research teams are more inclined to interview candidates with recognized credentials, especially for roles in NIH-funded studies or pharmaceutical trials.
According to industry surveys, certified research professionals report 20–35% higher entry salaries compared to uncertified peers. Moreover, the credential shortens the learning curve when onboarding into new roles. It proves you’ve already mastered core workflows—letting you skip beginner tasks and take on more impactful contributions.
Key Topics Covered in the Research Assistant Certification Exam
Understanding the exam content is critical—you can’t pass what you don’t fully understand. The Research Assistant Certification Exam evaluates both theoretical understanding and applied skills, spanning core scientific and operational areas. It's structured to test whether you can perform the day-to-day responsibilities of a research assistant while upholding regulatory and ethical standards.
Expect a mix of multiple-choice and scenario-based questions. The scenarios reflect real-world case studies, where you must select the correct action based on ICH-GCP, FDA, or IRB protocols. Successful candidates report that knowing the exam topics in depth—not just in definition—was essential to their pass rate. Below are the two foundational knowledge areas you'll be tested on.
Research Methodologies
A strong grasp of research methodologies is non-negotiable. This section evaluates how well you understand hypothesis formulation, study design types (qualitative vs. quantitative), and controlled vs. observational study structures. You’ll also be tested on concepts like randomization, blinding, and sampling techniques, as well as their implications for bias, error margins, and data validity.
It’s not enough to know definitions. You must demonstrate how to apply these concepts. For example, you may be asked how to improve the design of a flawed clinical study or explain the difference between cross-sectional and longitudinal studies in a given context. The Research Assistant Certification places strong emphasis on the ability to choose the right methodology for the right research question.
Data Analysis Techniques
Even at the assistant level, you must know how to interpret data. This section doesn’t require you to be a data scientist, but it does test your familiarity with descriptive statistics, basic inferential testing, and tools like SPSS or Excel. Expect questions on mean, median, mode, p-values, standard deviation, and basic confidence intervals.
You’ll also need to understand when to use a t-test vs. ANOVA, or what kind of graph best represents a certain data trend. Some programs might test your ability to recognize flawed or misleading graphs, too. Data analysis questions are often paired with short research summaries, requiring you to extract relevant patterns or flag inconsistencies.
How to Prepare for the Research Assistant Certification Exam
Preparation for the Research Assistant Certification isn’t about memorizing facts—it’s about developing job-ready skills. Candidates who pass on the first try follow structured plans, focusing not just on what to study but how. From content coverage to self-assessment strategies, your prep must simulate the exam experience closely while filling any knowledge gaps through active learning methods.
Use official study guides aligned with ICH-GCP and FDA standards, especially if your certification provider is CCRPS. Don’t rely on fragmented notes from college courses. Most importantly, avoid passive reading—opt for interactive tools like quizzes, flashcards, and mock tests. Below is a clear breakdown of how to build your study plan and test-readiness.
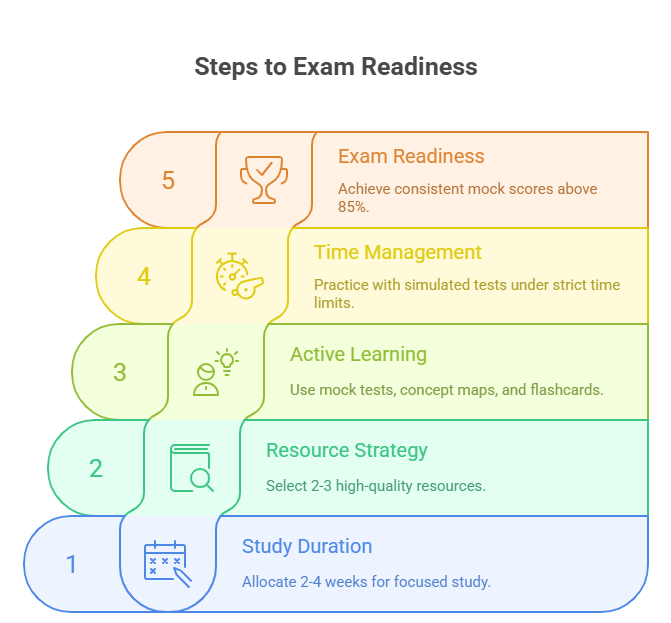
Study Schedule and Resources
Create a 4–6 week study timeline depending on your familiarity with core research topics. Break your schedule into daily 90-minute sessions, each focusing on a specific domain—methodology, data analysis, ethics, or compliance. Prioritize active recall over note-reviewing by using question banks and concept-mapping.
Recommended resources include CCRPS’s official Research Assistant Certification prep, NIH GCP training modules, and PubMed Central open-access journals for case examples. If you prefer structured instruction, platforms like Coursera or edX offer short research methods courses that complement certification prep. Keep your resource list lean—depth beats quantity.
Practice Exams and Mock Tests
Mock exams are your performance mirror. Take a full-length practice test weekly under timed conditions. Analyze every mistake—not just what you got wrong, but why you missed it. The best mock tests don’t just replicate question formats—they replicate mental pressure.
Aim to simulate exam fatigue. Take practice tests without notes, under a quiet, distraction-free environment, just like the actual testing center. Don’t just look for correct answers—learn the rationale behind each choice, especially on scenario-based questions. Repeating this routine for 3–4 weeks significantly boosts retention and confidence.
As your exam approaches, begin cycling through mixed-topic quizzes daily. Focus on tightening weak spots, not reinforcing what you already know. If your average score in mock exams is consistently above 85%, you’re likely ready to pass.
Who Should Take the Research Assistant Certification and Why
The Research Assistant Certification is not just for fresh graduates—it’s a powerful entry point for a variety of professionals aiming to enter or grow within the research industry. If your goal is to build a career grounded in clinical, academic, or scientific research, this credential can accelerate your transition with a globally respected validation of your readiness.
Whether you're switching industries or aiming to enhance your academic resume, here’s who benefits most:
Recent Graduates in Science or Healthcare Fields
Graduates with degrees in biology, chemistry, psychology, or public health often struggle to land research jobs without prior experience. This certification bridges that gap. It proves that you know how research operations work, from documentation and consent to data handling and regulatory alignment. Employers prefer candidates who don’t require basic training—and this is exactly what the certification signals.
Career Changers Entering the Research Field
Professionals transitioning from education, clinical roles, or general administration often need a formal entry credential. The Research Assistant Certification helps demonstrate that you’ve invested in learning ethical research conduct, study protocols, and documentation workflows. It also shows hiring managers you understand the industry’s expectations—even if your degree isn’t in a traditional science field.
International Professionals Seeking U.S.-Standard Training
For candidates outside the U.S. or Europe, certification offers a fast way to align with ICH-GCP and FDA protocols. This is especially valuable for remote contract work, research support positions, or CROs working across borders. The CCRPS program is globally recognized and gives international professionals a direct route into clinical and academic studies that follow U.S. guidelines.
Freelancers and Remote Job Seekers
Remote research assistant jobs often filter applicants through keyword-based hiring systems. Listing the Research Assistant Certification increases your odds of passing ATS filters and landing interviews. Freelancers who want to support clinical trial coordination, literature reviews, or regulatory documentation can use the certification to prove they’re not just assistants—they’re trained collaborators.
| Candidate Type | Why the Certification Helps |
|---|---|
| Recent Graduates (Biology, Psychology, Public Health) | Builds job-ready credibility and bridges experience gaps for research roles. |
| Career Switchers | Demonstrates formal training in ethics, compliance, and study protocols. |
| International Professionals | Aligns with ICH-GCP and FDA standards to qualify for U.S.-based research roles. |
| Freelancers & Remote Job Seekers | Improves visibility in ATS systems and proves capability for contract research support. |
Common Mistakes to Avoid During Preparation
Even strong candidates can fall short by making avoidable preparation errors. The Research Assistant Certification Exam requires a focused, strategy-driven approach—not just hard work. Knowing what not to do can be just as important as what to study. Common mistakes often stem from trying to do too much too fast or underestimating the exam’s depth.
From overloading your study plan to misjudging how much time is truly available during the exam, these errors can derail even the most motivated learners. Below are two critical pitfalls and how to dodge them effectively.
Overloading on Information
More resources do not mean more retention. One of the biggest mistakes is trying to consume too many books, courses, and videos in the hope of covering “everything.” This leads to surface-level understanding with little real recall. The human brain can’t internalize disorganized content without context or repetition.
Instead, pick 2–3 high-quality sources and stick with them. Focus on mastery, not volume. The certification exam doesn’t test how much you’ve read—it tests how well you understand and apply the content. Make sure you build knowledge actively: rewrite concepts in your own words, teach them out loud, or map workflows for retention. Eliminate content that doesn’t align directly with exam objectives.
Underestimating Exam Timing
Another silent killer is ignoring time management during practice. Many candidates believe they’ll have enough time to reason through questions carefully—until the clock starts ticking. The exam's design expects you to make quick decisions under pressure, especially with case-based questions that require multi-layered reasoning.
Start timing every quiz and mock test from day one. Practice finishing questions in under 90 seconds each, and make sure you’re not spending more than five minutes on any single scenario. Learn how to flag difficult items and return to them later. Pacing is a skill—and like any skill, it needs rehearsal.
A candidate might know the content cold and still fail because they couldn’t complete the test in time. Don’t let that be you. Treat time limits as part of the exam content—train for them the same way you train for knowledge.
| Mistake | Consequence | Prevention Strategy |
|---|---|---|
| Resource Overload | Surface-level understanding | Focus on fewer, deeper study materials |
| Ignoring Practice Tests | Poor time management during exam | Weekly full-length mock exams |
| Passive Studying | Low retention | Use recall-based tools and timed drills |
| Skipping Scenario Practice | Failure in applied questions | Focus on real-world case question types |
| Underestimating Exam Depth | False confidence, poor outcomes | Study GCP/FDA protocols actively |
How the CCRPS Research Assistant Certification Accelerates Career Development
The CCRPS Research Assistant Certification does more than validate knowledge—it directly expands your career potential. In research-intensive sectors like biotech, pharmaceuticals, and academic institutions, hiring managers prefer candidates with recognized credentials that prove operational readiness. This certification meets that demand with globally standardized content, aligned to FDA and ICH-GCP guidelines.
By earning the CCRPS credential, you show employers you're already trained in regulatory protocols, ethics documentation, and data handling processes. That alone can fast-track job interviews and reduce onboarding time. Many research organizations favor CCRPS-certified candidates because they understand documentation best practices, consent protocols, and subject protections from day one.
Professionals who hold this certification are frequently considered for promotions into roles like Clinical Research Coordinators or Regulatory Specialists. In entry-level hiring rounds, the certification becomes a competitive edge—especially in environments flooded with recent graduates who lack hands-on research exposure. For contract roles, freelance monitoring, or research assistantships in grant-funded projects, it serves as proof of compliance and capability.
If you're aiming to transition into research from another field, this certification bridges that gap—instantly signaling credibility. Recruiters often use certifications like CCRPS as filters in ATS (Applicant Tracking Systems), which means it can even determine whether your resume is seen by a human at all.
In short, the CCRPS Research Assistant Certification is more than a piece of paper. It’s a direct investment in your ability to access higher-paying roles, climb faster within research institutions, and take on specialized responsibilities sooner than peers without formal training.
Frequently Asked Questions (FAQs)
-
The CCRPS Research Assistant Certification is one of the most recognized and globally standardized credentials for entry into the research field. It’s designed to align with FDA and ICH-GCP guidelines, giving candidates a real-world understanding of research compliance, ethics, data handling, and regulatory processes. Unlike generic certifications, CCRPS focuses specifically on clinical and academic research environments. This makes it ideal for those seeking roles in medical, pharmaceutical, or academic institutions. Additionally, many employers actively seek CCRPS-certified applicants because they require minimal training and can contribute from day one. If you’re serious about building a long-term research career, this certification is an industry-approved starting point.
-
The difficulty of the exam depends on your background. For candidates new to research or regulatory work, the CCRPS Research Assistant Certification exam can feel rigorous because it requires understanding both theoretical principles and applied protocols. However, the program is designed to be accessible, even for those without a science degree. With proper preparation—such as reviewing the course modules, taking mock tests, and understanding core areas like research design and ethics—you can pass confidently. Most students who follow a structured study plan complete the course and exam in 2–4 weeks. The exam is open-book but scenario-based, testing real-world understanding.
-
The CCRPS exam covers a range of topics essential for professional research assistants. Key domains include research methodologies, ethical conduct in human subject research, FDA and ICH-GCP compliance, data collection and reporting, informed consent protocols, and clinical trial documentation. The exam also includes case-based questions designed to test your ability to apply this knowledge in real-world situations. You’ll be expected to interpret scenarios, identify violations, or choose the correct process in ethically sensitive situations. This ensures that certified professionals are not just familiar with textbook theory but can perform under regulatory expectations in research settings.
-
Most candidates prepare for the CCRPS Research Assistant Certification in 2 to 4 weeks, depending on their prior knowledge and daily study time. The course is self-paced, and CCRPS provides a structured module system that you can follow easily. If you dedicate 1–2 hours per day, you can complete the core content in under 3 weeks and still leave time for revision and mock tests. The key is consistency and sart review: focusing on high-yield topics, understanding real-world scenarios, and practicing under timed conditions. Candidates from non-science backgrounds may need closer to 4–5 weeks of preparation.
-
Yes, the CCRPS Research Assistant Certification is widely recognized by employers in healthcare, academia, and clinical research sectors. The program is aligned with FDA, NIH, and ICH-GCP standards, which are considered global benchmarks in research ethics and practice. Many job listings for research assistant or coordinator roles include certifications like CCRPS as preferred or even required qualifications. Employers value this certification because it ensures new hires already understand ethical guidelines, informed consent procedures, and basic compliance workflows—reducing training costs and risk. Holding this certification often helps applicants bypass entry-level filters and move directly to interviews.
-
Yes, many employers hire entry-level candidates with no prior experience if they have earned a recognized certification like CCRPS’s Research Assistant Certification. This credential demonstrates that you understand how research operations function, even if you haven’t yet worked on a real study. It covers essential topics like GCP compliance, research design, data handling, and subject safety—allowing you to confidently step into your first research support role. If paired with a relevant degree or coursework (like biology or psychology), the certification significantly increases your chances of getting hired, especially in academic labs and clinical research organizations.
-
Absolutely. The CCRPS Research Assistant Certification is recognized by global employers and based on international guidelines like ICH-GCP and FDA protocols. This makes it a strong fit for candidates across North America, Europe, Asia, and the Middle East. Many international research firms and CROs value this certification because it shows alignment with global standards. Whether you're seeking remote roles or planning to work in a multinational research facility, the credential strengthens your resume and proves you're qualified to support regulated studies. It’s particularly valuable in countries where research regulation is rapidly expanding and certified staff are in high demand.
Conclusion: Successfully Navigating the Research Assistant Exam
Passing the Research Assistant Certification isn’t just about getting a credential—it’s about stepping into research with confidence, clarity, and capability. With a structured plan, targeted resources, and the right certification provider—like CCRPS—you can gain the foundational knowledge and applied skills needed to thrive in today’s competitive research environment.
Focus on mastering core concepts, understanding regulatory standards, and practicing under exam-like conditions. Don’t chase every resource—go deep on a select few that align with ICH-GCP and FDA guidelines. Most importantly, treat this certification as more than a checkbox. It’s your entry point to jobs with real impact—from assisting in clinical trials to contributing to groundbreaking academic work.
Commit to preparation. Avoid common pitfalls. Practice strategically. And when exam day comes, walk in knowing you’ve done what most candidates won’t—you prepared with intent.