Clinical Research Certification North Dakota: Everything You Need to Know for 2025-2026
North Dakota’s clinical research field is expanding quietly but steadily — backed by Sanford Health, Essentia Health, and major academic research at North Dakota State University. As the state diversifies into biotech and pharmacology trials, certified professionals are increasingly sought after. If you want to build a career in clinical trials or regulatory compliance, obtaining a Clinical Research Certification is your fastest route to credibility and opportunity.
Learn how to earn certification, compare career paths to nearby regions like South Dakota and Minnesota, and discover why the CCRPS Clinical Research Certification remains the gold standard for 2025–2026.
1. North Dakota’s Clinical Research Ecosystem and Certification Demand
Clinical research in North Dakota focuses on oncology, rare diseases, and community-based trials. Institutions such as Sanford Health and UND’s School of Medicine are driving over 300 active studies across Fargo and Bismarck.
The CCRPS Clinical Research Certification aligns with global standards like ICH-GCP and FDA 21 CFR Part 312, ensuring candidates meet sponsor expectations. Similar to Oregon’s model, North Dakota’s research network increasingly prefers certified staff for compliance-heavy positions like Clinical Research Associate (CRA) and Clinical Research Coordinator (CRC).
Graduates from Rhode Island and Tennessee have already proven the career jump such certifications enable.
North Dakota Clinical Research Certification Outlook (2025–2026)
| Key Factor | 2025–2026 Data |
|---|---|
| Projected Job Growth | 15 % increase (2025–2026) |
| Average CRA Salary | $77,000 – $105,000 annually |
| Entry-Level CRC Pay | $55,000 – $62,000 |
| Top Hiring Cities | Fargo, Bismarck, Grand Forks |
| Major Employers | Sanford Health, UND Research Institute, Essentia Health |
| Course Duration | 6–8 weeks (self-paced) |
| Exam Format | Online 100-question proctored |
| Passing Score | 75 % |
| Renewal Cycle | Every 3 years (24 CE credits) |
| Accreditation | ICH-GCP, HIPAA, FDA 21 CFR |
| Remote Opportunities | 40 % hybrid/remote roles |
| Major CROs Hiring | IQVIA, ICON, Parexel, PPD |
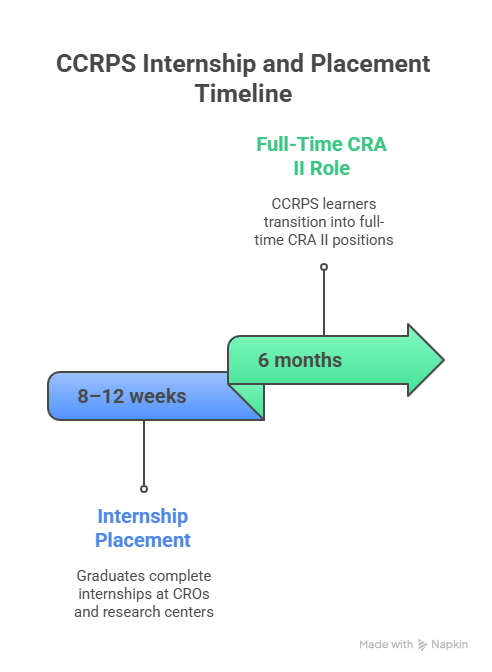
| Internship Duration | 8–12 weeks |
| Career Ladder | CRC → CRA → PM → Director |
2. Steps to Earn Your Clinical Research Certification in North Dakota
To earn your certification through CCRPS:
1️⃣ Enroll in the CCRPS Clinical Research Certification — fully online and compliant with international standards.
2️⃣ Complete learning modules in protocol development, informed consent, and data validation.
3️⃣ Pass the 100-question online exam.
4️⃣ Apply for internships using the CCRPS placement network, modeled after programs in Pennsylvania.
5️⃣ Renew every 3 years with CE credits.
The process mirrors pathways in Virginia and Washington, ensuring consistent standards nationwide.
3. Career Growth and Salary Insights in North Dakota
With limited competition and expanding CRO presence, certified professionals in North Dakota are earning competitive salaries comparable to urban states. Entry-level CRCs start at $55K, while CRAs reach $100K+ after 2–3 years of experience.
Certification from CCRPS enhances your eligibility for global remote roles and sponsor monitoring jobs, similar to Oklahoma’s CRA pathway. Employers recognize CCRPS training for its FDA-aligned rigor and real-case assessment methodology.
What’s Holding You Back from Certification in North Dakota?
4. Internship and Placement Opportunities
Through the CCRPS Internship Portal, graduates access 8–12-week placements with CROs and research centers like Sanford Health and ICON. Interns gain hands-on experience with data integrity checks, source documentation, and IRB coordination.
These internship structures resemble the pathways in Oregon and South Carolina, where CCRPS learners transitioned into full-time CRA II roles within 6 months.
5. Why CCRPS Certification Is the Best Choice for North Dakota
CCRPS offers a globally recognized certification, fully aligned with FDA, ICH-GCP, and HIPAA frameworks. Its emphasis on applied case studies and regulatory precision gives learners a career edge across all clinical trial phases.
Professionals from Wisconsin and West Virginia show measurable advancement post-certification — proving CCRPS’s cross-state reliability.
CCRPS’s continuous learning platform also helps professionals maintain compliance and CE credits without pausing their career.
6. FAQs: Clinical Research Certification North Dakota
-
Yes — hospitals and CROs recognize it as an international standard.
-
Anyone with a degree in life sciences, healthcare, or pharmacy is eligible.
-
6–8 weeks on average through self-paced online study.
-
Yes — placements are available via the CCRPS Internship Portal.
-
Comparable to national averages; installment options available.
-
Every 3 years with continuing-education credits.
-
Entry-level CRCs earn $55K, while CRAs reach $100K+.