How to Become a Clinical Trial Manager
Introduction: So You Want to Be a Clinical Trial Manager? Here’s the (Not-So-Secret) Formula
If you’re fascinated by groundbreaking medical discoveries but also have an unexplainable love for spreadsheets and protocols, congratulations—you might just have what it takes to become a clinical trial manager (CTM). This role is where scientific discovery meets project management, and guess what? The demand is skyrocketing in 2025.
Whether you’re eyeing this career path for its six-figure salary potential (yes, we see you, financially savvy folks) or because you genuinely want to be part of life-saving research, this guide will walk you through everything you need to know. From education and certification to must-have skills and little-known industry secrets, let’s break down the blueprint to becoming a top-tier clinical trial manager.
Before managing trials, it’s essential to understand the fundamentals of clinical trials and phases of clinical trials to ensure compliance and efficiency throughout the research process.
What is a Clinical Trial Manager, and Why is This Role So Important?
A Clinical Trial Manager (CTM) is responsible for overseeing clinical research studies, ensuring compliance with regulatory standards, and managing teams of researchers and coordinators. They play a vital role in the development of new drugs, treatments, and medical devices.
With the pharmaceutical and biotech industries booming, the demand for skilled CTMs has surged. In fact, the Bureau of Labor Statistics projects a 13% job growth in this field by 2030—way above the average for most professions.
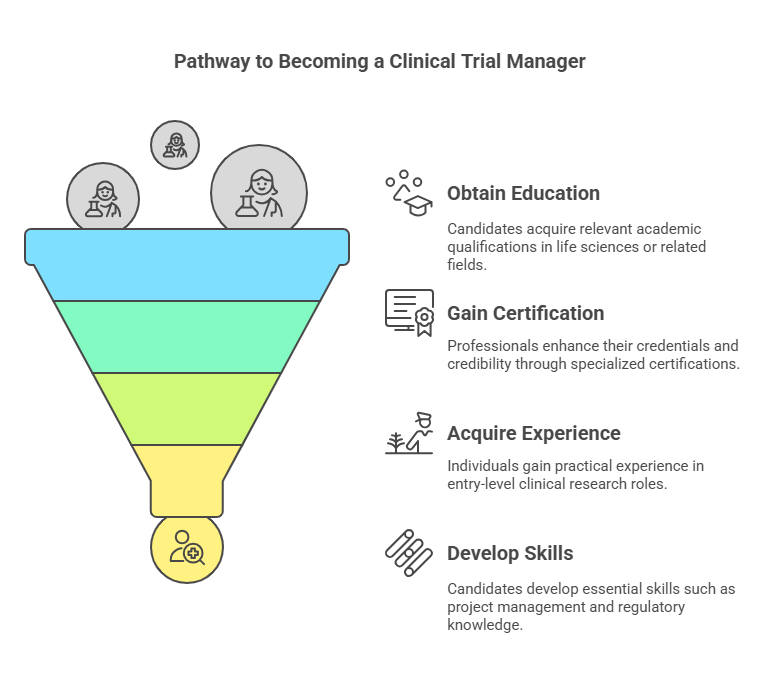
Steps to Becoming a Clinical Trial Manager
Becoming a Clinical Trial Manager (CTM) requires a mix of education, experience, and certification. The first step is obtaining a bachelor's degree in life sciences, clinical research, or a related field. Some professionals also pursue a master's degree for advanced knowledge. Practical experience in clinical research is crucial, often gained through entry-level roles like Clinical Research Coordinator (CRC) or Clinical Research Associate (CRA). Earning certifications, such as the Clinical Trial Manager Certification, demonstrates expertise and enhances job prospects. Additionally, strong leadership, organizational, and analytical skills are necessary for managing clinical trials effectively.
1. Obtain the Right Education
While there’s no single “magic degree” for becoming a CTM, most professionals start with a background in:
Life Sciences (Biology, Chemistry, Biochemistry, or Pharmacology)
Clinical Research or Health Sciences
Nursing or Medicine
Public Health or Healthcare Administration
Many employers prefer candidates with at least a bachelor’s degree, while a master’s degree in clinical research or a related field can give you a competitive edge.
2. Get Certified – The Key to Career Acceleration
To stand out in the job market, earning a Clinical Trial Manager Certification is crucial. These certifications prove your expertise and boost your credibility. The top certifications include:
Good Clinical Practice (GCP) Certification – A must-have for regulatory compliance.
Clinical Research Coordinator (CRC) Certification – Great for transitioning into a managerial role.
Clinical Research Associate (CRA) Certification – Ideal for those with monitoring experience.
Pharmacovigilance Certification – Perfect if you’re interested in drug safety.
Medical Monitor Certification – Designed for professionals overseeing clinical trials.
Where to get certified? CCRP Certification Programs offer some of the most recognized certifications in the industry.
3. Gain Hands-On Experience
Most CTMs start as clinical research coordinators (CRCs) or clinical research associates (CRAs) before stepping into management. Here’s how to build your experience:
Work at research hospitals, CROs (Contract Research Organizations), or pharmaceutical companies.
Gain experience in regulatory compliance, data management, and team leadership.
Get familiar with Clinical Trial Management Systems (CTMS) and software like Oracle Clinic.
4. Master the Essential Skills
Being a CTM isn’t just about knowing clinical research inside out. You’ll need:
Project Management Expertise – Juggling multiple trials is part of the job.
Regulatory Knowledge – FDA, EMA, and ICH-GCP guidelines are your best friends.
Data Analysis Skills – Understanding trial data is crucial.
Communication & Leadership – Managing teams and reporting findings is daily business.
Attention to Detail – A single oversight can delay an entire study.
If you're wondering about earning potential, check out how much clinical trial managers make to get insights into salary ranges and career growth opportunities.
10 Lesser-Known Facts About Clinical Trial Management
1. Clinical Trials Date Back to the 1700s
The first known clinical trial was conducted in 1747 by Dr. James Lind to study the effects of citrus fruits on scurvy among sailors. His research laid the foundation for modern clinical trials.
🔗 National Library of Medicine
2. The Majority of Clinical Trials Fail
Approximately 86% of clinical trials fail to move from Phase I to final approval due to inefficacy, safety concerns, or financial constraints.
🔗 Biotechnology Innovation Organization
3. Clinical Trial Managers Must Understand AI & Big Data
AI-driven patient recruitment and big data analytics are revolutionizing clinical trials, making it crucial for Clinical Trial Managers to be tech-savvy.
🔗 Forbes
4. Global Clinical Trials Face Cultural and Ethical Challenges
Clinical trials conducted internationally must comply with varying ethical standards, regulatory guidelines, and cultural sensitivities, making global trials complex.
🔗 World Health Organization
5. Some Clinical Trials Pay Participants Thousands of Dollars
Healthy volunteers in Phase I trials can earn between $2,000 and $10,000 per study, depending on duration and risk factors.
🔗 CenterWatch
6. Remote and Decentralized Trials Are the Future
COVID-19 accelerated the shift towards virtual clinical trials, reducing costs and improving patient participation rates.
🔗 Nature
7. The U.S. Is Home to the Most Clinical Trials
Over 30% of all clinical trials worldwide are conducted in the United States, with China and Europe following closely.
🔗 ClinicalTrials.gov
8. Only 5% of Eligible Patients Participate in Clinical Trials
Despite the need for diverse data, only 5% of eligible patients enroll in clinical trials due to lack of awareness and accessibility barriers.
🔗 American Cancer Society
9. The Clinical Research Market Will Be Worth Over $80 Billion by 2030
With the rise in personalized medicine, the clinical research industry is projected to surpass $80 billion by 2030.
🔗 Grand View Research
10. Clinical Trial Managers Can Work Remotely
Many companies now offer remote roles for Clinical Trial Managers, allowing them to oversee trials from anywhere in the world.
🔗 LinkedIn Jobs
Final Thoughts: Your Path to Becoming a Certified Clinical Trial Manager
The clinical research industry is growing rapidly, and clinical trial managers are more in demand than ever. If you’re serious about breaking into this field, get the right education, gain hands-on experience, and earn your certification through trusted programs like CCRP’s Clinical Research Training.
Ready to take the next step? Check out CCRP’s top-rated courses: