Clinical Trial Auditing & Inspection Readiness: CRA’s Expert Guide
Clinical trial audits and regulatory inspections don’t “start” when someone shows up on-site — they start months earlier in the daily decisions that either preserve traceability or silently destroy it. This CRA expert guide shows you exactly how to build inspection readiness into monitoring: what to verify, what evidence must exist, where sites usually fail, and how to correct risk before it becomes a finding. You’ll also get a practical readiness checklist and a decision-focused approach to data integrity, safety documentation, and essential documents that consistently holds up under scrutiny.
1) What inspection readiness really means for a CRA
Inspection readiness is not a binder that “looks good” — it’s the ability to prove that trial conduct, safety handling, and data are reliable, complete, and traceable from protocol intent to patient-level evidence. As a CRA, your value isn’t that you find problems; it’s that you detect systemic risk early and push the site into sustainable controls. That starts with knowing how the protocol’s scientific logic translates into verifiable execution, especially around endpoint-critical data defined by primary vs secondary endpoints, blinding controls explained in blinding types and importance, and allocation rigor clarified in randomization techniques. Readiness also depends on whether the site’s documentation culture can withstand forensic review — which is why CRAs must push disciplined source strategy aligned with CRF best practices and regulatory doc control aligned with managing regulatory documents for CRCs. If you’re early in your monitoring career, treat this as the “how to think” layer behind the role described in CRA roles, skills, and career path: inspections reward CRAs who enforce evidence, not opinions.
In real inspections, regulators don’t care that a process exists — they care that it produced the right outcomes for this participant, on this date, under this protocol version. That means your monitoring must continuously test three questions: (1) Did the site do what the protocol required? (2) Can the site prove it with source, logs, and contemporaneous notes? (3) If something went wrong, was it detected, assessed, reported, and corrected appropriately? The most damaging findings typically occur when sites “did the right thing” but cannot prove it, or when they documented something that isn’t truly supported by source. If you want to prevent that, you have to focus your time where inspections focus: endpoint-critical assessments, eligibility evidence, dosing and visit windows, safety reporting discipline, and essential document control — all of which become far easier to manage when you understand operational roles across the site from the lens of CRC responsibilities and certification and the compliance mindset embedded in pharmacovigilance essentials.
| Readiness Control Area | What a CRA Verifies | Inspection Failure Pattern | Evidence to Collect / Expect | Corrective Action (Fast + Durable) |
|---|---|---|---|---|
| Protocol version control | Only current protocol/tools used | Mixed-version execution | Version log + dated training | Cutover checklist + retire old tools |
| ICF version + signatures | Correct ICF, proper timing | Consent after procedures | Dated signatures + narrative notes | Pre-procedure consent gate |
| Eligibility traceability | Each criterion backed by source | Ineligible enrollment | Eligibility checklist + source refs | Decision tree + PI sign-off |
| Randomization gate | All pre-reqs met before rand | Randomized “too early” | Gate checklist + system timestamps | No-rand rule until evidence complete |
| Visit window compliance | All visits in window or documented | Undocumented window misses | Window calendar + notes | Scheduling alerts + rescue slots |
| Endpoint-critical assessments | Timing + completeness + method | Endpoint data not credible | Source packets + instrument records | Endpoint watchlist + QC cadence |
| AE collection quality | Complete narratives | Vague AEs, missing dates | AE source, onset/offset, grading | AE interview script + review step |
| SAE reporting timeliness | SAE reported within required time | Late or unreported SAE | SAE report timestamps + correspondence | Same-day escalation rule |
| Causality/Severity/Outcome | Consistent + justified | Inconsistent grading | PI assessment + documentation | PI review triggers + training |
| Concomitant meds | Prohibited meds identified | Hidden exclusion meds | Med list + reconciliation notes | Med review at each visit |
| IP accountability | Receipt/dispense/return reconciled | IP discrepancies | IP logs + counts + temperature logs | Dual verification + monthly recon |
| Temperature excursions | Excursions managed + documented | Unaddressed excursions | Temp logs + excursion forms | Excursion workflow + escalation |
| Lab kit handling | Correct tubes + timing + shipping | Specimen rejection | Processing logs + courier receipts | Timed checklists + training sign-off |
| Vendor portal workflows | Data entered + reconciled | Missing vendor data | Portal audit trails | Portal task ownership + backups |
| Source-to-CRF consistency | CRF values trace to source | “Orphan” CRF values | Source location map | Source indexing + QC |
| ALCOA+ expectations | Attributable/legible/contemp/orig/accurate | Backdated notes, unclear authorship | Signed/dated entries + audit trails | Documentation training + spot checks |
| Corrections policy | Corrections compliant | White-out, missing initials | Correction policy + examples | Standard correction method training |
| Delegation of Authority | Tasks match delegation + training | Undelegated procedures performed | DOA log + training matrix | Monthly DOA reconciliation |
| Training documentation | Role-based, protocol-specific | Generic “attendance” logs | Training map + competency proof | Role-based training checklist |
| Regulatory binder completeness | Essential docs current and filed | Missing approvals/versions | Binder QC log | Binder QC calendar + owner |
| IRB/EC approvals | Approval prior to implementation | Unapproved changes used | Approval letters + effective dates | Change control gate |
| Protocol deviations handling | Captured, assessed, reported | Under-reporting deviations | Deviation log + notifications | Weekly deviation review |
| CAPA documentation | Root cause + prevention evidence | Repeat issues | CAPA forms + effectiveness checks | CAPA owner + timeline + verification |
| Monitoring visit documentation | Findings tracked to resolution | Unresolved repeat findings | Follow-up letters + action trackers | Issue tracker + deadlines |
| Query management | Queries resolved with proof | Queries closed without source support | Query responses + source refs | QC before query closure |
| Data privacy / access controls | Appropriate access + audit trails | Unauthorized access | User access logs | Role-based access review |
| Close-out readiness | All outstanding items resolved | Last-minute scramble | Close-out checklist | Rolling close-out plan |
CRA mindset: If it isn’t documented, it didn’t happen — and if it isn’t traceable, it isn’t credible.
2) Build an inspection-ready site system, not a “pre-audit panic”
The most inspection-ready sites aren’t perfect — they’re controlled. They detect risk early, document decisions contemporaneously, and keep essential documents in a state that could be reviewed tomorrow without embarrassment. As a CRA, you create readiness by engineering routines into site operations, not by delivering a once-a-quarter “be ready” speech. Start by building the site’s daily evidence chain around documentation discipline: what belongs in source, what belongs in CRF, and how the two connect, using the mindset in CRF definition, types, and best practices as your anchor. Then align the site’s regulatory hygiene to an essentials-first approach like the one in managing regulatory documents for CRCs, because incomplete approvals and missing versions create the kind of “easy findings” that destroy sponsor confidence.
Next, make the site operationally fluent in the protocol’s scientific logic so they prioritize correctly under pressure. If the protocol’s success depends on endpoint timing, tie the site’s behaviors to the clarity in primary vs secondary endpoints so everyone understands why certain fields are non-negotiable. If blinding is present, build simple guardrails grounded in blinding types and importance so accidental unblinding doesn’t become a credibility crisis. If randomization is involved, align the “randomize only when evidence is complete” culture to the rationale behind randomization techniques explained clearly, because inspectors will examine whether allocation steps were executed consistently, not whether the staff intended to follow them.
Finally, embed safety rigor as a daily reflex. Inspection failure often comes from weak AE narratives and late SAE reporting — not because teams don’t care, but because they don’t have repeatable collection habits. Reinforce safety literacy through the practical lens in what is pharmacovigilance and connect it to role expectations across the team, including a clear division of responsibilities described in CRC responsibilities and certification and the CRA’s oversight accountability described in CRA roles and skills. When you build readiness as a system, inspections become a review of controlled behavior — not a hunt for surprises.
3) Audit-proof monitoring: what to source-verify every visit
A CRA’s strongest protection is not charisma; it’s verification discipline. Regulators and auditors pull threads: one subject, one endpoint, one AE, one randomization, one protocol deviation — and then they test whether every connected record is consistent. Your monitoring should mirror that logic. Every visit, you should be verifying “high-liability” elements: eligibility evidence, consent timing, endpoint-critical assessments, investigational product accountability, and safety documentation. When these are stable, most other problems become manageable.
Start with consent and eligibility because they are foundational. If consent timing is wrong or eligibility is not provable, the entire subject’s data becomes vulnerable. Then verify protocol-critical assessments through the endpoint lens clarified in primary vs secondary endpoints and ensure your site understands which assessments drive interpretability. If you see inconsistent assessment timing or missing supporting documentation, treat it as an integrity risk, not a clerical issue — especially in designs where placebo comparison or blinding are central, because execution variance can distort interpretation in the way explained in placebo-controlled trials and expose operational blind vulnerabilities described in blinding importance.
Next, verify data capture quality through source-to-CRF logic. If the CRF says something happened, you need to see where that truth lives in source — and whether it was documented contemporaneously. Use the discipline described in CRF best practices to detect “orphan values,” inconsistent dates, or copy-forward behaviors that look harmless but fail under inspection. Then review safety as a narrative-quality exercise, not a checkbox: ensure AEs have clear onset/offset, severity, seriousness criteria, causality assessment, outcome, and any action taken — and align the team’s thinking to pharmacovigilance essentials so they don’t under-document or delay escalation.
Finally, verify essential documents and delegation realism. Inspectors frequently uncover that tasks were performed by staff not listed on delegation logs or not trained for that protocol version. Push the site to maintain an inspection-ready binder system consistent with regulatory documents management for CRCs and help them build role clarity aligned with CRC responsibilities. This is how you reduce the probability of a “simple” administrative gap becoming an inspection-level finding.
Which area causes the most audit risk at your sites?
Pick one. This reveals where your monitoring should go deeper (and where you should build stronger controls).
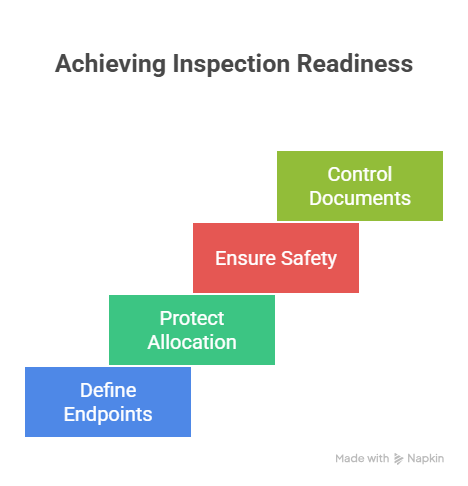
4) Risk-based inspection readiness: where expert CRAs spend their time
Inspection readiness becomes dramatically easier when you stop treating all data equally. Expert CRAs prioritize by risk to credibility: if a failure would invalidate eligibility, compromise blinding, undermine endpoint integrity, or create safety non-compliance, it deserves disproportionate attention. This is the logic behind risk-based monitoring — but in inspections, it becomes unforgiving because regulators naturally gravitate to high-impact pathways: inclusion/exclusion logic, endpoint drivers, randomization, unblinding, and safety. Your job is to make those pathways robust and provable.
Start with endpoints and interpretability. If you don’t know which assessments drive trial conclusions, you can’t protect them. Use the clarity in primary vs secondary endpoints to define an “endpoint watchlist” at each site: the specific data points that must be complete, timely, and traceable. Then ask: where could reality drift? Tight visit windows, competing clinic workflows, staffing variability, or patient adherence issues. This is where you coordinate with CRC teams whose operational responsibilities are mapped in CRC responsibilities and certification, because readiness is ultimately created at the task level — who does what, when, and how it’s documented.
Next, protect allocation integrity. In randomized trials, inspectors may verify that randomization occurred only after eligibility was proven and that randomization procedures were applied consistently. Tie your monitoring gates to the principles explained in randomization techniques so the team respects why deviations here are not “minor.” If blinding exists, treat it like a brittle system: ensure access controls and unblinding steps align to the vulnerabilities described in blinding importance. And if a placebo-controlled design is in play, remember that “small” execution inconsistencies can cascade into interpretability issues in exactly the way discussed in placebo-controlled trials.
Finally, protect safety. Inspection findings often arise when safety documentation is inconsistent, late, or incomplete — especially when the story doesn’t match the medical record. Reinforce robust AE collection behaviors using pharmacovigilance essentials and anchor site corrections to durable documentation controls recommended in CRF best practices. When safety and endpoint integrity are stable, your remaining inspection-readiness work becomes a matter of disciplined essential documents control, which should follow the structured approach in managing regulatory documents and the role clarity described in CRA career responsibilities.
5) Inspection day playbook: how to lead the site without creating new risk
When an inspection notice arrives, your first move is to prevent “panic editing” — last-minute retro-documentation that creates audit-trail anomalies and credibility damage. The goal is controlled transparency: organize evidence, clarify roles, and ensure the site can quickly retrieve documentation without improvising stories. As a CRA, you become a calm operations leader who protects the integrity of what already exists.
Begin with a controlled readiness sweep that prioritizes the exact records inspectors commonly request: consent forms and dates, eligibility evidence, endpoint-critical source, AE/SAE documentation, IP accountability, delegation/training, protocol/ICF versions, and key correspondence. Tie your sweep to a traceability mindset anchored in CRF best practices so the site doesn’t confuse “data entered” with “data supported.” Then align the regulatory binder and essential documents to a structure consistent with regulatory documents management, because scattered documents waste time and increase the risk of inconsistent answers under pressure.
Next, coach the team on how to answer questions. Inspectors test whether staff understand their responsibilities and whether their explanations align with documented procedures. This is why role clarity matters: reinforce operational boundaries using CRC responsibilities and make sure PI and sub-investigator expectations are clear. If the inspection touches allocation procedures, ensure the staff can explain the logic behind them with the confidence that comes from understanding randomization techniques and, if applicable, can describe how they protect the blind as outlined in blinding importance. If questions relate to endpoints or timing, ensure the team can connect the “what” to the “why” using endpoint clarity — because inspection interviews often expose whether a site is executing with understanding or just following checklists.
Finally, manage document requests as a controlled workflow. Create a request log that tracks what was requested, when it was provided, and where the source came from. This prevents “multiple versions” of the truth from being handed over. Ensure AE/SAE packages are complete and consistent with the medical record, using the safety mindset from pharmacovigilance essentials. And if you identify real gaps, handle them transparently: document the issue, correct forward where possible, and ensure CAPA is practical and preventive — the same quality thinking that underpins inspection durability in quality-centric roles such as those described in QA specialist roadmap. Inspection readiness is not about perfection; it’s about credibility — and credibility is built by controlled behavior and provable records.
6) FAQs
-
The most common reason is lack of traceability: the site may have done the right thing, but the documentation doesn’t prove it clearly, consistently, and contemporaneously. Inspectors expect CRF values to map to source and safety narratives to align with the medical record, which is why strengthening workflows using CRF best practices and documentation discipline from regulatory documents management is often the fastest readiness upgrade.
-
Prioritize what threatens credibility: consent timing, eligibility evidence, endpoint-critical assessments, randomization/blinding controls, and safety documentation. Anchor your “what matters most” list in the trial’s endpoint logic using primary vs secondary endpoints, allocation rigor using randomization techniques, and blind protection using blinding importance, then build repeatable site controls around them.
-
Stop panic edits, initiate a controlled readiness sweep, and organize retrieval workflows. Focus on essential documents and subject-level packages (consent, eligibility, key visits, AEs/SAEs, IP). Ensure roles and responsibilities are clearly understood across the team using CRC responsibilities and CRA expectations in CRA role framework.
-
Teach sites to document safety as a complete story: onset/offset, seriousness, severity, causality, action taken, outcome, and supporting evidence. Implement consistent AE interview questions and same-day escalation rules, grounded in the operational mindset of pharmacovigilance essentials. Most safety findings come from incomplete narratives or late action, not from rare medical complexity.
-
Amendments create risk when sites operate in mixed versions — old tools, old windows, old procedures — even when the new version is “trained.” Prevent this with a cutover mindset: retire old documents, update workflows, retrain by role, and document effective dates in the binder structure recommended by regulatory document management. Mixed-version execution is a classic “avoidable finding” that CRAs can prevent with strong change control.
-
Treat readiness as a daily system: traceability-first documentation, risk-based verification, stable endpoint protection, and proactive safety handling. The most effective CRAs build this culture through consistent monitoring expectations and site coaching aligned with CRF best practices, operational role clarity from CRC responsibilities, and professional CRA execution standards in CRA roles and skills.