Clinical Research Certification Alaska: Everything You Need to Know for 2025-2026
Alaska may seem far removed from America’s major research hubs, but it’s quickly becoming a frontier for decentralized and community-based clinical research. With increased federal grants for rural healthcare innovation, and rising partnerships between tribal health organizations, academic centers, and CROs, demand for certified professionals is growing fast.
Earning your Clinical Research Certification in Alaska through CCRPS opens career opportunities with hybrid CROs, remote data teams, and telehealth-linked trial sponsors — even if you’re hundreds of miles from the nearest research hospital.
1. Alaska’s Clinical Research Landscape (2025–2026 Overview)
Alaska’s remote geography has become its greatest advantage in modern research. The global pivot to remote and hybrid clinical trials has made Alaska an active test site for digital data collection, patient outreach, and telemedicine-based study management.
According to NIH rural health grant data, over $180 million in funding has been allocated for Alaskan trials between 2024–2026, covering fields like cardiology, endocrinology, mental health, and infectious disease.
Organizations such as the Alaska Native Tribal Health Consortium (ANTHC) and University of Alaska Anchorage (UAA Research Institute) collaborate with CCRPS-trained professionals to ensure ethical oversight, data compliance, and GCP adherence.
Graduates from CCRPS Clinical Research Certification programs are already managing site coordination remotely from Anchorage and Fairbanks, proving that distance is no longer a limitation. Explore regional parallels in Clinical Research Certification Colorado and Clinical Research Certification Nevada to see how CCRPS-certified professionals are redefining access.
2. Why Alaska Is Ideal for Certified Clinical Researchers
While smaller in population, Alaska’s per-capita funding for clinical research is among the top five in the U.S.. The region’s focus on telemedicine, Indigenous health studies, and infectious disease prevention creates sustained demand for GCP-trained coordinators and auditors.
With employers like ANTHC and Providence Health, certified professionals often enjoy greater independence and remote flexibility than those in larger states. The ability to handle regulatory submissions and decentralized patient management has made CCRPS graduates the top choice for sponsor audits and CRO oversight.
Learn how this remote-first model compares to Clinical Research Certification Hawaii, Clinical Research Certification Oregon, and Clinical Research Certification California.
3. Salary Outlook & Career ROI
Salary potential in Alaska is surprisingly strong due to limited competition and federal funding incentives. CCRPS-certified CRAs and CRCs often command 10–15% higher salaries than peers without certification.
Average annual compensation:
Clinical Research Assistant: $52,000–$62,000
CRC: $60,000–$78,000
CRA I–II: $80,000–$112,000
Clinical Project Manager: $115,000–$140,000
Employers value CCRPS graduates for their hands-on skills in risk-based monitoring, data integrity, and audit-readiness. You can explore salary parallels in Clinical Research Certification Florida, Clinical Research Certification Georgia, and Clinical Research Certification Delaware.
What’s Holding You Back from Starting Your CCRPS Certification in Alaska?
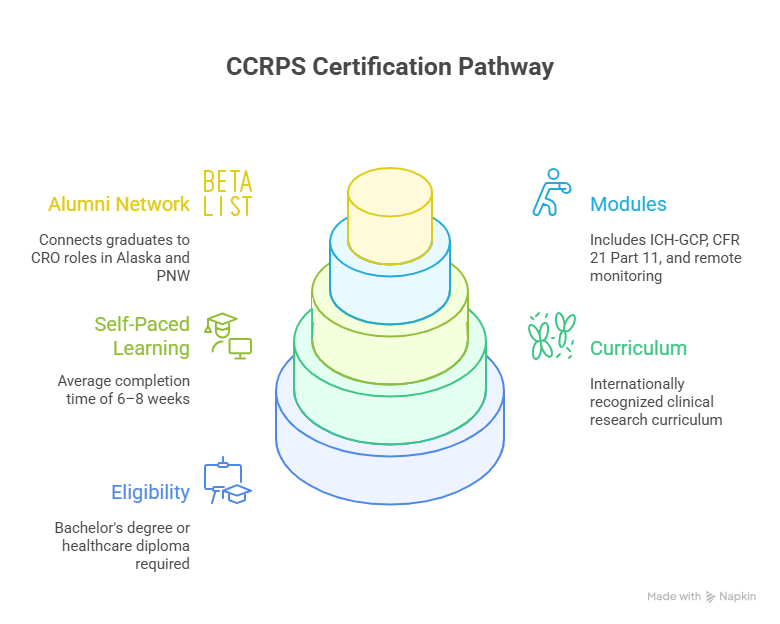
4. Certification Pathway & Eligibility
The CCRPS Clinical Research Certification for Alaskan professionals follows the same internationally recognized curriculum trusted by top sponsors and CROs.
Eligibility requirements:
Bachelor’s degree or healthcare diploma
Understanding of patient documentation or lab data management
Internet access (for remote learning modules)
CCRPS’s program is self-paced, averaging 6–8 weeks, and includes modules in ICH-GCP, CFR 21 Part 11, and Remote Monitoring Standards. Students gain access to the CCRPS Alumni Network, which connects graduates to hybrid CRO roles across Alaska and the Pacific Northwest.
Learn more about academic alignment from Clinical Research Certification Indiana and Clinical Research Certification Michigan.
5. Challenges and Opportunities for Alaska’s Research Workforce
While Alaska’s decentralized trials create exciting roles, professionals face several hurdles:
Limited local mentors or CRO offices
Travel constraints for hybrid monitoring
Harsh weather conditions affecting site access
Smaller trial networks requiring cross-role skills
CCRPS addresses these with simulation-based learning, regulatory mock audits, and remote internship assistance through national CRO partners. Graduates gain the skills to work remotely for U.S. or global sponsors, making geography irrelevant.
The same flexible framework powers success stories in Clinical Research Certification Louisiana and Clinical Research Certification Kentucky.
6. FAQs – Alaska Clinical Research Certification (2025–2026)
-
A bachelor’s degree or equivalent healthcare experience, such as lab or nursing work, is required.
-
Typically 6–8 weeks, depending on your study pace.
-
Yes. CCRPS offers partnerships with U.S.-based CROs that accept remote trainees.
-
Anchorage and Fairbanks lead, with CRA salaries up to $112,000.
-
Yes. CCRPS is recognized by sponsors and CROs globally for its GCP-compliant curriculum.
-
Yes. Many CCRPS graduates manage site monitoring for CROs based in Seattle, San Francisco, and Dallas.
-
CRC, CRA I, CRA II, Regulatory Associate, and Data Manager.
-
Yes. CCRPS maintains an active alumni network providing mentorship, career guidance, and job placement support.