Clinical Research Certification Nevada: Everything You Need to Know for 2025-2026
Nevada’s clinical research ecosystem is compact but high-velocity: Las Vegas anchors multi-site Phase II–IV work while Reno pivots to hybrid and decentralized models. If you’re aiming for certification that converts into paid roles (CRC, CTA, Regulatory), your edge is job-ready competence, state-aware networking, and cross-state leverage from day one. Use the strategy below to build skills that matter to Nevada PIs and CROs, while tapping proven playbooks from neighboring states like Utah, Oregon, Washington, and California-adjacent markets.
1) Nevada’s 2025–2026 hiring reality — and what “job-ready” actually means
Nevada sites hire for immediate utility: coordinators who can consent patients correctly, prepare source/visit worksheets that pass monitoring review, and enter EDC data cleanly with minimal query bounce-back. Hiring managers skim resumes for signals like GCP/ICH proof, adverse event reporting fluency, protocol deviation prevention, and comfort with patient scheduling across casino-shift lifestyles. Strengthen those signals with targeted reading from high-performing state guides such as North Carolina, Pennsylvania, Tennessee, and Virginia to copy what works in similar markets.
Las Vegas sites prioritize screen-fail reduction, tight SAE triage paths, and sponsor-friendly visit cadence. Reno/Carson City clinics push cross-coverage skills across cardiometabolic and oncology. To stand out, attach a mini-portfolio to applications: two de-identified source templates (Screening + Visit 1), a sample EDC entry log with audit trail highlights, and a corrective-action memo that shows how you would handle a protocol deviation. Support each artifact with CCRPS-aligned references pulled from state pages like New York, New Jersey, and Rhode Island so screeners see you’ve done the homework.
2) The certification path that actually gets you hired in Nevada
Your certification should translate directly into visit-ready performance. Prioritize a program that drills consent walkthroughs, AE/SAE classification, and eSource/EDC practice reps. Reinforce with CCRPS state playbooks that prove portability: South Carolina, South Dakota, Wisconsin, and West Virginia. Employers respond to patterns: candidates who can show repeatable, compliant visit flow from screening to EOS.
Translate theory into outputs hiring managers can hold. Build a mini-portfolio that includes: (1) Nevada-specific pre-screen checklist tailored to casino-shift workers; (2) consent conversation script with teach-back prompts; (3) de-identified EDC entry plus an audit trail excerpt; (4) a protocol-deviation CAPA memo that demonstrates root-cause depth. Link these skills to external signals in your resume by referencing CCRPS guidance from North Dakota, Ohio, and Oklahoma pages to show portability across sponsor networks.
For newcomers pivoting from hospitality, allied health, or admin roles, anchor your transition with CCRPS CRA/CRC-focused articles like the CRA guides for Oregon, Pennsylvania, and South Dakota. Then, present Nevada-specific value: show you can schedule around atypical work hours, coordinate transport in extreme heat, and manage multilingual consent support—these are real pain points Nevada sites face weekly.
3) Get hired faster: externships, scripts, and small wins that compound
Externship strategy: target multi-therapeutic clinics in Las Vegas for breadth and seek hospital-affiliated sites in Reno for deeper SOP exposure. Offer micro-commitments (8–12 hours/week for 4–6 weeks) with defined deliverables: “I will build de-identified source worksheets, pre-screen 30 candidates, and close five open queries.” Back this with proof that you understand sponsor expectations by reading cross-market pages such as Vermont, Washington, and New Mexico.
Outbound script for PIs/Managers:
Opener: “I’m CCRPS-trained in GCP/ICH and eSource/EDC. I can reduce monitor findings by building clean source for your busiest protocol within two weeks.”
Proof: attach the three artifacts mentioned earlier and a one-page KPI plan (consent audit, query rate, visit turnaround).
Close: “I’m available Tue/Thu evenings and Saturdays—ideal for Nevada scheduling realities.”
Stack small wins: de-identify a screen-fail analysis for one study and convert insights into a pre-screen checklist. Document how you cut query rates from 12% to 4% by improving vitals timing and lab result checks. Package the improvement with links to state benchmarking pages like Pennsylvania, Rhode Island, and Wisconsin to show the improvement isn’t a one-off.
What’s your biggest roadblock to getting hired in Nevada clinical research?
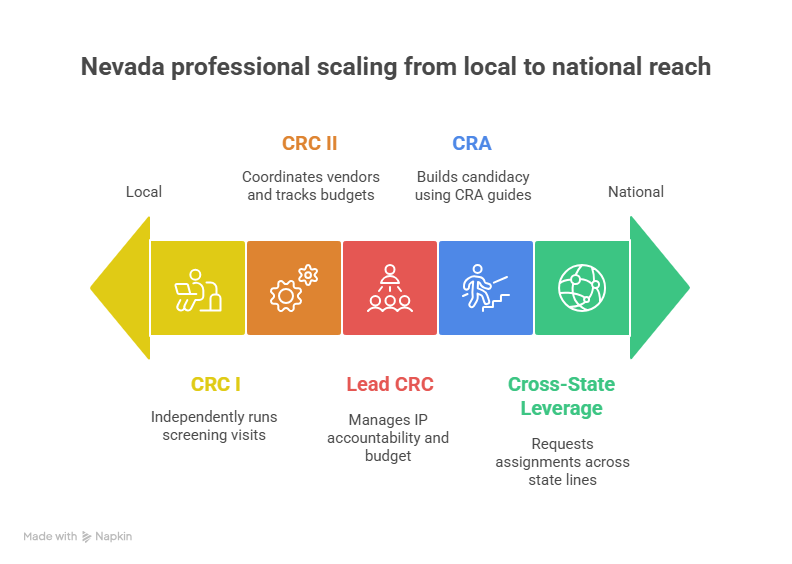
4) Salaries, career ladders, and cross-state leverage (how Nevada professionals scale)
Year-one CRCs usually land between $48k and $66k depending on city and overtime windows. The quickest jumps aren’t from negotiating— they’re from measurable quality: low query rates, zero consent deviations, and fast monitor close-outs. Track these in a personal KPI log and reference them during quarterly reviews. To benchmark compensation and role scope, review nearby markets like Utah, Oregon, and Washington to gauge cross-offers and remote CTA options.
The career ladder is straightforward if you make yourself easy to trust. CRC I → CRC II often happens within 10–12 months when you can independently (1) run Screening/Visit 1, (2) process reg amendments quickly, and (3) pre-empt monitor findings. From there, expand your value with vendor coordination (couriers/home-health), IP accountability, and basic budget tracking; those skills set up Lead CRC or Regulatory Lead outcomes. If you prefer the sponsor side, build your CRA candidacy using CCRPS CRA guides from Oklahoma, Oregon, and Pennsylvania. The through-line: demonstrate reliability with documentation and timelines, then ask for stretch protocols where you can own a visit series.
Cross-state leverage is a quiet advantage in Nevada. Many Las Vegas study networks also operate in California, Arizona, and Utah. Use that footprint to request temporary assignments that broaden your therapeutic exposure. When applying, seed your resume with links to market pages like New York, New Jersey, and Virginia to show your understanding of sponsor expectations across regions.
5) The Nevada job-search playbook: a 30-60-90 plan that converts
Days 1–30 — Build undeniable proof. Complete certification aligned to GCP/ICH and high-utility site tasks. Create three artifacts: (1) Nevada-tuned consent script, (2) pre-screen checklist with lab timing and shift-worker accommodations, (3) CAPA sample for a hypothetical deviation. Practice eSource/EDC with a sandbox. As you assemble materials, borrow phrasing and KPI ideas from strong state pages like Pennsylvania, Rhode Island, and Wisconsin.
Days 31–60 — Secure an externship with deliverables. Offer time-boxed help and promise specific outcomes: “I’ll build source for Protocol X, pre-screen 30 candidates, and close 10 queries.” Track each micro-win in a simple dashboard and ask the PI for a one-paragraph testimonial. Reference assets from South Carolina, South Dakota, and West Virginia pages to position your results within broader norms.
Days 61–90 — Convert the externship into paid work. Ask for spillover responsibilities: dose accountability logs, courier coordination, and reg binder maintenance. Present a simple ROI: “My checklist cut screen fails by 30%; my visit worksheets reduced monitor findings by half.” While interviewing, demonstrate portability via CCRPS CRA/CRC guides for Oregon, Oklahoma, and South Dakota. Employers hire speed and certainty; your artifacts and numbers provide both.
6) FAQs — Nevada-specific answers that remove guesswork
-
Yes—if you present proof of competence, not just certificates. Deliver a mini-portfolio: consent script, pre-screen checklist, and a de-identified EDC log with an audit trail. Offer micro-commitments to Las Vegas or Reno sites with clear deliverables. For structure, model your plan on CCRPS state guides like Utah and Oregon and adapt to Nevada’s scheduling realities.
-
Prioritize GCP/ICH, consent mastery, AE/SAE fundamentals, and eSource/EDC fluency. Don’t chase alphabet soup—show visit-ready skill tied to Nevada pain points: shift-worker consent, transportation coordination, heat-sensitive IP handling. Learn sponsor expectations by scanning high-signal pages like Washington and Virginia.
-
Design evening/weekend visit blocks, use SMS confirmations, and pre-arrange transport where possible. Build a consent flow that survives noisy environments by using teach-back prompts and quiet rooms. Package these tactics into your application materials. For templates and benchmarking, review Pennsylvania, New Jersey, and New York.
-
Two levers: clean source and fast action-item closure. Build visit worksheets from the schedule-of-assessments, time-stamp every procedure, and pre-link labs to windows. After each monitoring visit, close items within 7 days and document CAPA. For examples of what “good” looks like, mine CCRPS pages for North Carolina, Rhode Island, and Wisconsin.
-
Pick the quickest path to measurable wins. If you’re people-strong and detail-oriented, CRC accelerates faster in Nevada because you can show immediate visit impact. If you’re document-driven, CTA/Reg roles build sponsor trust and open remote options. Either path benefits from CRA thinking; use CRA guides for Oregon, Oklahoma, and Pennsylvania.
-
Walk in with a 3-deliverable plan: source build, pre-screen volume, and query closures. Track metrics weekly and present a one-page “before/after.” Ask your PI for a testimonial paragraph you can paste into applications. Align your approach to norms you see in South Carolina, South Dakota, and West Virginia.
-
Top section: “Core Competence” with GCP/ICH, consent, EDC tools, and SAE basics. Next: three bullet caselets (pre-screen checklist, CAPA memo, visit worksheet). Then: externship outcomes with numbers. Attach a link to a portfolio folder and pepper in state-context links such as Utah, Oregon, and Washington to communicate portability.