Global Pharmacovigilance Regulations
Imagine if your morning headache pill suddenly turned you into a superhero. Sounds fun, right? Well, in reality, unregulated medications can do more harm than good. From mild allergic reactions to life-threatening conditions, the importance of pharmacovigilance (PV) cannot be overstated. With billions spent on drug development and safety, ensuring medications don’t cause unintended chaos is a global responsibility.
So, how do international agencies manage this? Buckle up as we dive into the intricate world of Global Pharmacovigilance Regulations and explore who’s keeping an eye on the medicines you trust.
What is Pharmacovigilance?
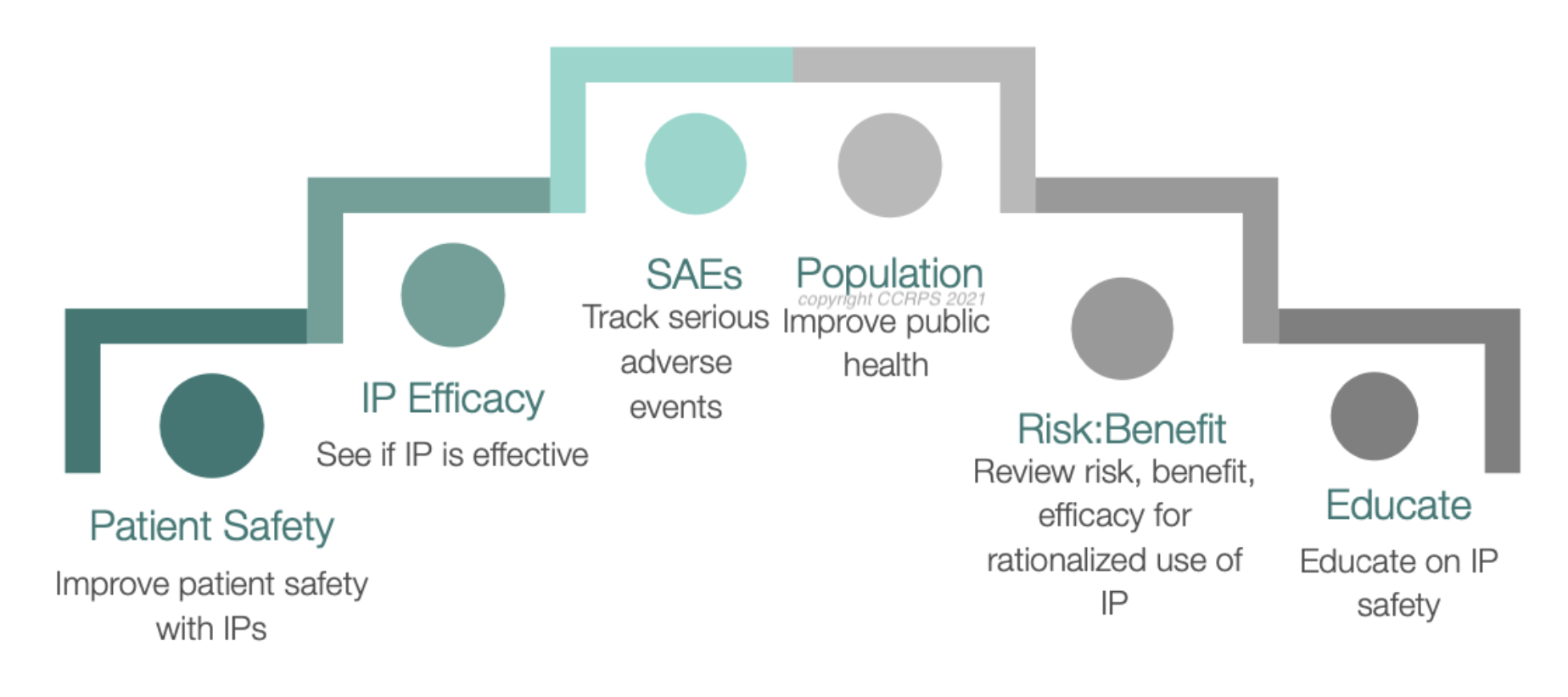
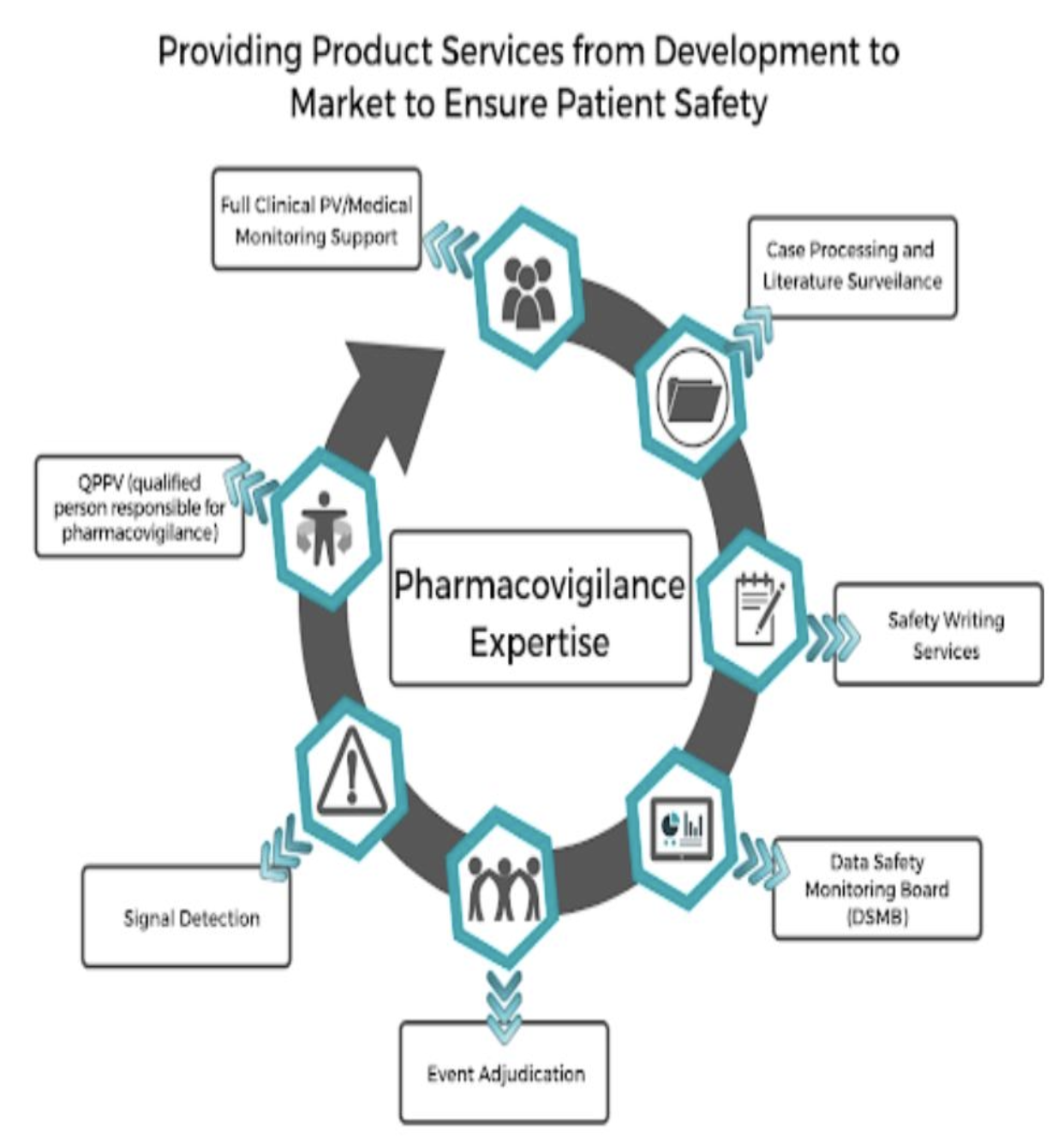
Pharmacovigilance (PV), often referred to as drug safety monitoring, is the science and practice of detecting, assessing, understanding, and preventing adverse drug reactions (ADRs) and other drug-related risks. In simple terms, PV ensures that the medicines people take are safe and effective—not just when they are first approved but throughout their entire lifecycle.
Why Is Pharmacovigilance So Important?
Imagine this: A brand-new medication enters the market after extensive testing, but once millions of people start using it, unexpected side effects emerge. What happens next? Pharmacovigilance experts step in! They analyze real-world data to determine if the benefits of the drug outweigh its risks. If necessary, they update safety guidelines, issue recalls, or restrict drug usage.
Without PV, the global healthcare system would be at risk of dangerous drug reactions, medical errors, and preventable deaths. This is why pharmacovigilance is one of the most critical pillars of modern medicine.
Key Objectives of Pharmacovigilance
Pharmacovigilance is more than just reacting to adverse drug effects—it’s a proactive approach to ensuring drug safety worldwide. The major goals include:
✅ Identifying previously unknown ADRs – Continuous drug monitoring helps detect new adverse reactions.
✅ Improving patient safety – PV professionals assess the risks associated with medications and take preventive action.
✅ Developing risk management strategies – Pharmaceutical companies use PV data to modify dosages, issue warnings, or withdraw unsafe drugs.
✅ Enhancing international cooperation – Global regulatory authorities work together to create unified drug safety guidelines.
With the rapid advancements in AI, blockchain, and real-world evidence analysis, pharmacovigilance is evolving faster than ever in 2025.
Who Regulates Pharmacovigilance? Key Global Authorities
Pharmacovigilance isn’t just handled by a single organization—it’s a global effort. Various international and national regulatory bodies collaborate to ensure drug safety across borders. Let’s break down the most important authorities:
1. International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH)
📍 Established: 1990
📍 Covers: U.S., EU, Japan, and other major regions
📍 Purpose: Standardizing drug safety regulations across countries
The ICH harmonizes PV guidelines to ensure consistent drug safety measures worldwide. Its key document, ICH Good Clinical Practice (ICH GCP), sets standards for ethical and scientific clinical research.
ICH’s Role in 2025
🔹 AI-driven drug monitoring systems are being incorporated into ICH guidelines.
🔹 The organization is focusing on AI-generated drug risk assessments to predict potential ADRs before market approval.
2. European Medicines Agency (EMA)
📍 Region Covered: 27 EU Member States
📍 Primary Regulations: Good Pharmacovigilance Practices (GVP)
The EMA ensures that drugs in the European Union are monitored throughout their lifecycle—from clinical trials to post-market use. Their EudraVigilance database, launched in 2023, has significantly improved real-time tracking of ADRs.
EMA’s Role in 2025
🔹 The agency is now using big data analytics to improve early ADR detection.
🔹 AI models are automating drug safety reports, reducing delays in safety warnings.
3. U.S. Food and Drug Administration (FDA)
📍 Region Covered: United States
📍 Key Department: Center for Drug Evaluation and Research (CDER)
The FDA plays a central role in approving and monitoring pharmaceutical products in the U.S. Within the FDA, CDER’s Office of Surveillance and Epidemiology is responsible for overseeing pharmacovigilance.
FDA’s Role in 2025
🔹 The Sentinel Initiative, an advanced AI-powered system, is now detecting ADRs faster than ever.
🔹 Blockchain-based record-keeping is being used in clinical trials to improve data security.
4. Pharmaceuticals and Medical Devices Agency (PMDA) - Japan
📍 Region Covered: Japan
📍 Key System: SAKIGAKE Fast-Track System
Japan’s PMDA accelerates drug approval for innovative medicines while ensuring rigorous safety monitoring.
PMDA’s Role in 2025
🔹 Real-world data analysis is now a part of Japan’s post-market surveillance system.
🔹 AI-powered drug safety analytics predict ADRs before they impact the market.
5. Marketed Health Products Directorate (MHPD) - Canada
📍 Region Covered: Canada
📍 Function: Monitors and regulates health product risks
MHPD consists of six specialized bureaus dedicated to monitoring adverse drug reactions and making regulatory decisions.
MHPD’s Role in 2025
🔹 AI-driven ADR database has been launched to track drug safety alerts in real time.
🔹 Enhanced collaboration with the U.S. and EU improves cross-border pharmacovigilance efforts.
The Role of Technology in Pharmacovigilance (2025 Trends)
1. AI and Machine Learning in PV
🧠 AI can analyze millions of patient records in seconds, detecting ADR patterns much faster than traditional methods.
🔹 Predictive analytics help identify drugs that may need additional monitoring.
🔹 AI-driven chatbots are assisting doctors and patients in reporting ADRs instantly.
2. Blockchain for Drug Safety
🔗 Blockchain is now ensuring tamper-proof data sharing between regulatory agencies and pharmaceutical companies.
🔹 This eliminates data fraud in clinical trials.
🔹 Ensures greater transparency in drug safety reporting.
3. Wearable Health Devices in PV
⌚ Smartwatches and wearable medical devices can now track real-time biometric changes to detect ADRs.
🔹 Continuous monitoring means doctors receive instant alerts when a patient experiences a serious side effect.
Career Opportunities in PV
With global drug safety regulations expanding, demand for PV professionals is at an all-time high! Some of the most in-demand PV careers include:
✅ Drug Safety Associate – Investigates ADR reports and ensures compliance with regulations.
✅ Clinical Research Coordinator – Manages clinical trials and ensures ethical drug testing.
✅ Medical Monitor – Oversees safety monitoring during clinical trials.
For those interested in pursuing a career in PV,
Conclusion
Pharmacovigilance is essential to ensuring global drug safety, and as technology advances, so do PV strategies. If you’re interested in a career in pharmacovigilance, clinical research, or drug safety monitoring, CCRPS offers expert-led online certification programs to help you get started.
Explore Courses for Clinical Research Career
Courses Available: