How to Land a Career in PV?
So, you want to get into pharmacovigilance (PV), but you don’t want to spend your life in a white coat mixing mysterious potions? Good news! PV isn’t about brewing chemicals; it’s about making sure the ones already on the market don’t send people on unexpected (and unpleasant) adventures. If you’re the kind of person who secretly enjoys analyzing Yelp reviews for patterns, you might just love PV.
But how do you actually land a job in this field? Well, grab your coffee (or energy drink—no judgment), and let’s dive into the ultimate guide to breaking into the world of pharmacovigilance in 2025!
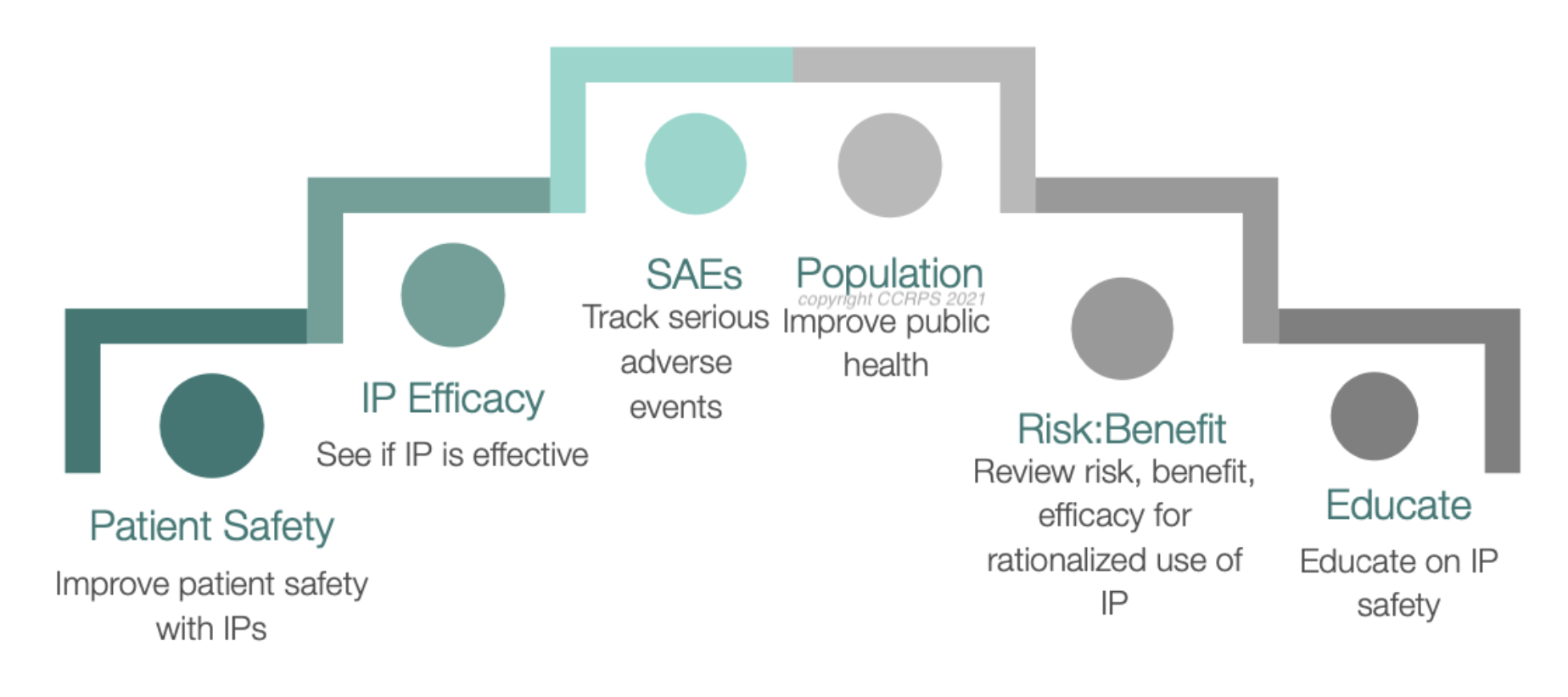
What is Pharmacovigilance (PV)?
Pharmacovigilance is the science of detecting, assessing, understanding, and preventing adverse effects or any drug-related problems. In simpler terms: you make sure medications don’t harm people and that regulatory authorities stay happy.
PV is a booming field, with the global drug safety market expected to reach $15 billion by 2027. This demand translates to excellent career opportunities for those who are passionate about data analysis, medical safety, and regulatory compliance.
Education Requirements
Pharmacovigilance (PV) is an exciting field that focuses on monitoring and ensuring drug safety, but you don’t necessarily need to be a medical doctor to enter it. However, a strong background in healthcare, life sciences, or data analytics is highly recommended.
Educational Paths to Enter PV
Bachelor’s Degree – Most entry-level pharmacovigilance jobs require a bachelor’s degree in fields such as:
Pharmacy (BPharm, PharmD)
Nursing (BSN, RN, LPN)
Medicine (MD, DO)
Health Sciences (Public Health, Biomedical Sciences)
Dentistry or Veterinary Medicine (DDS, DVM)
Master’s or PhD in PV or Clinical Research – Higher degrees can open doors to specialized and leadership roles within pharmacovigilance. These programs focus on drug development, risk assessment, regulatory compliance, and data analysis.
Certifications in Drug Safety & PV – If you don’t have a traditional healthcare degree, or you want to strengthen your credentials, certifications in pharmacovigilance, drug safety, and clinical trials can enhance your resume. Some well-known programs include those from CCRPS, DIA (Drug Information Association), and ICH (International Council for Harmonization).
Recommended Skills for Pharmacovigilance Careers
Since pharmacovigilance involves analyzing drug safety data and ensuring compliance with regulations, possessing the following skills can improve your job prospects:
Data Analysis & Management – Since PV involves handling large amounts of patient and drug safety data, familiarity with tools like Excel, SAS, R, Python, and SQL is beneficial.
Regulatory Knowledge – Understanding global drug safety regulations like FDA (US), EMA (Europe), and ICH guidelines helps in compliance roles.
Attention to Detail – Small mistakes in adverse event reports or regulatory documentation can have serious consequences.
Communication Skills – Clear writing and verbal skills are necessary to draft safety reports, signal detection summaries, and regulatory documents.
PV Specializations: Finding Your Niche
Pharmacovigilance isn’t a one-size-fits-all career. There are different roles within the field, depending on your expertise and interests.
Common Pharmacovigilance Specializations
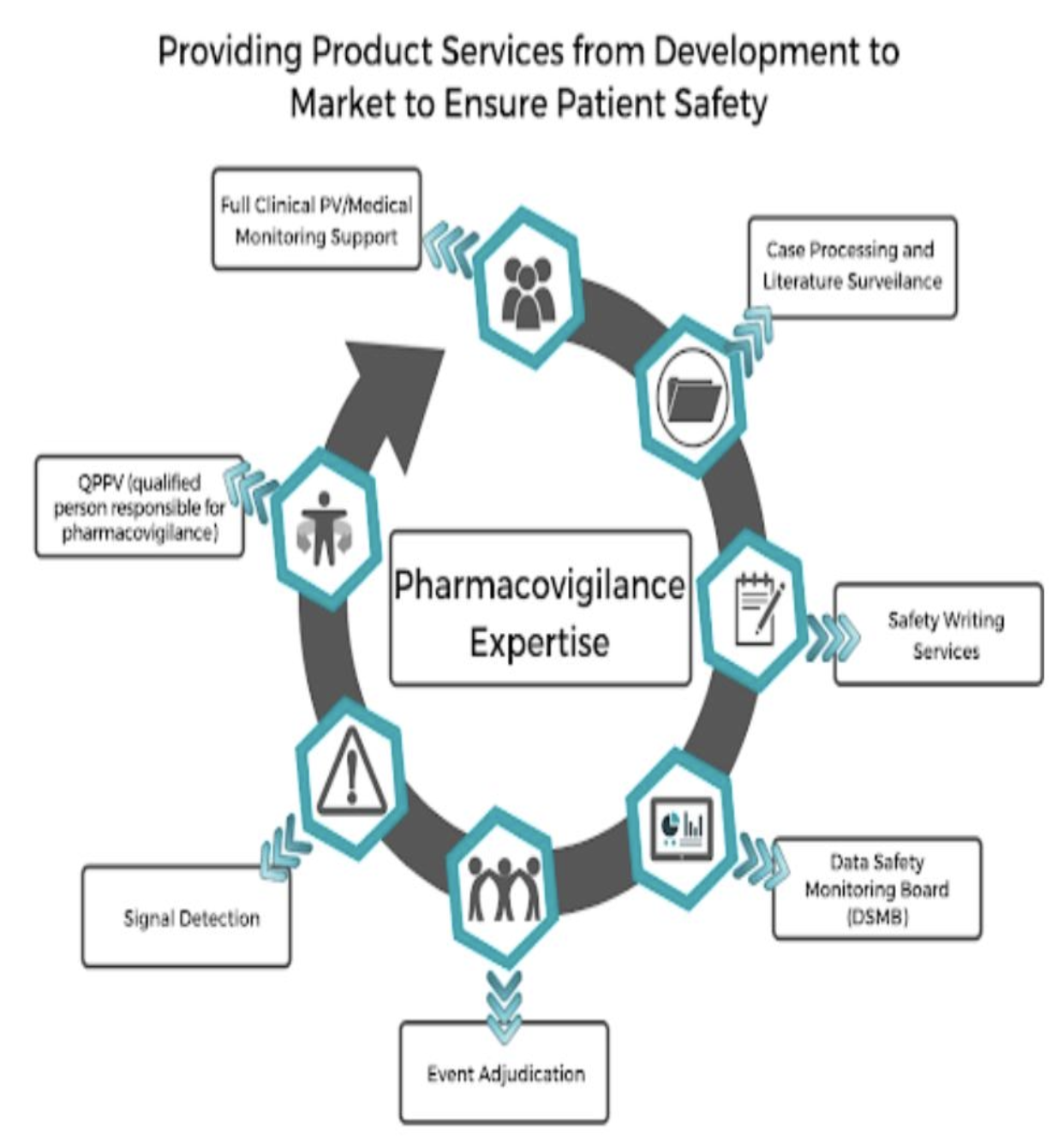
Case Processing & Data Entry – This role involves collecting, reviewing, and recording adverse drug reactions (ADRs) reported by patients, doctors, or researchers.
Medical Safety Writing – Specialists in this area write scientific and regulatory reports regarding drug safety data, clinical trials, and risk assessments.
Quality & Compliance Management – This role ensures that pharmaceutical companies follow international drug safety guidelines, reducing the risk of legal penalties.
Signal Detection & Risk Management – Professionals in this field identify patterns in adverse event reports to detect early warning signals of potential drug risks.
Where to Find Pharmacovigilance Jobs?
There are many sectors that hire PV professionals, including:
Pharmaceutical Companies – The biggest employers in pharmacovigilance, responsible for monitoring the safety of their drugs.
Contract Research Organizations (CROs) – These companies outsource pharmacovigilance activities for multiple pharma clients.
IT & Knowledge Process Outsourcing (KPOs) – These organizations manage data processing and analysis related to PV.
Hospitals & Universities – Academic and research institutions conduct clinical trials and require PV professionals for monitoring.
Regulatory Agencies – Agencies like the FDA (US), EMA (Europe), and MHRA (UK) hire pharmacovigilance officers to ensure public safety.
Entry-Level PV Roles & Salaries
If you’re just starting out, here are some entry-level pharmacovigilance jobs, along with their average salaries (as of 2025):
Pharmacovigilance Specialist – $68,995/year
Responsibilities: Reporting and recording adverse drug reactions (ADRs).
Pharmacovigilance Associate – $90,557/year
Responsibilities: Ensuring that clinical trials and post-market monitoring meet safety regulations.
Pharmacovigilance Scientist – $123,499/year
Responsibilities: Analyzing data, assessing risks, and compiling reports for regulatory authorities.
Source: https://www.ziprecruiter.com/Salaries/Entry-Level-Pharmacovigilance-Salary--in-Ontario
How to Land a PV Job?
To break into pharmacovigilance, follow these three steps:
Step 1: Build a Strong Resume
Your CV should highlight:
✔ Education and certifications in pharmacovigilance or related fields
✔ Internships or research projects in drug safety
✔ Technical skills like data analysis and regulatory knowledge
Step 2: Gain Practical Experience
Apply for internships, apprenticeships, or trainee roles in pharmacovigilance.
Volunteer for medical writing, clinical research, or regulatory documentation projects.
Complete online certification programs (e.g., from CCRPS or DIA) to enhance your skills.
Step 3: Apply to Jobs & Network
Use LinkedIn to connect with pharmacovigilance professionals.
Attend pharma and drug safety conferences (like DIA Global Annual Meeting).
Contact PV recruiters or apply through job boards (Indeed, Glassdoor, Pharmajobs, etc.).
Explore Courses for Clinical Research Career
Courses Available:
Conclusion
Pharmacovigilance is one of the fastest-growing sectors in the pharmaceutical industry, with companies and regulatory bodies increasingly focusing on drug safety and patient well-being.
For those interested in this field, enrolling in industry-recognized certification programs from CCRPS can provide the necessary skills and knowledge to succeed in this competitive industry.