Pharmacovigilance Positions and Careers in Clinical Research
What is Pharmacovigilance?
Drugs are an integral part of healthcare. New medications are constantly developed and tested by pharmaceutical companies to be put on the market. However, according to the American Society of Pharmacovigilance, adverse drug events account for: 1 million emergency department visits, 2.2 million hospital admissions, 3.5 million physician office visits, and $136 billion in U.S. health care annually. Thus, it is paramount that drugs are as safe they can be.
Pharmacovigilance (PV), or drug safety, is the study of a drug’s adverse reactions. PV professionals work in varying specialities to ensure that a drug is safe and tested before it is consumed by the masses. In this article, we will look at the different ways PV professionals contribute to drug safety in clinical research settings.
Why is PV Important?
A drug needs to be approved by the appropriate regulatory body before going on the market. In the U.S., the FDA determines if a drug is ready for the market. Before a drug can apply for FDA approval, it must undergo three phases of clinical trials. Every proceeding phase will involve more subjects and be more risky. Thus, it is important for the clinical research team and pharmaceutical company to be sure that a drug is safe before proceeding with the next phase.
What Roles are in PV?
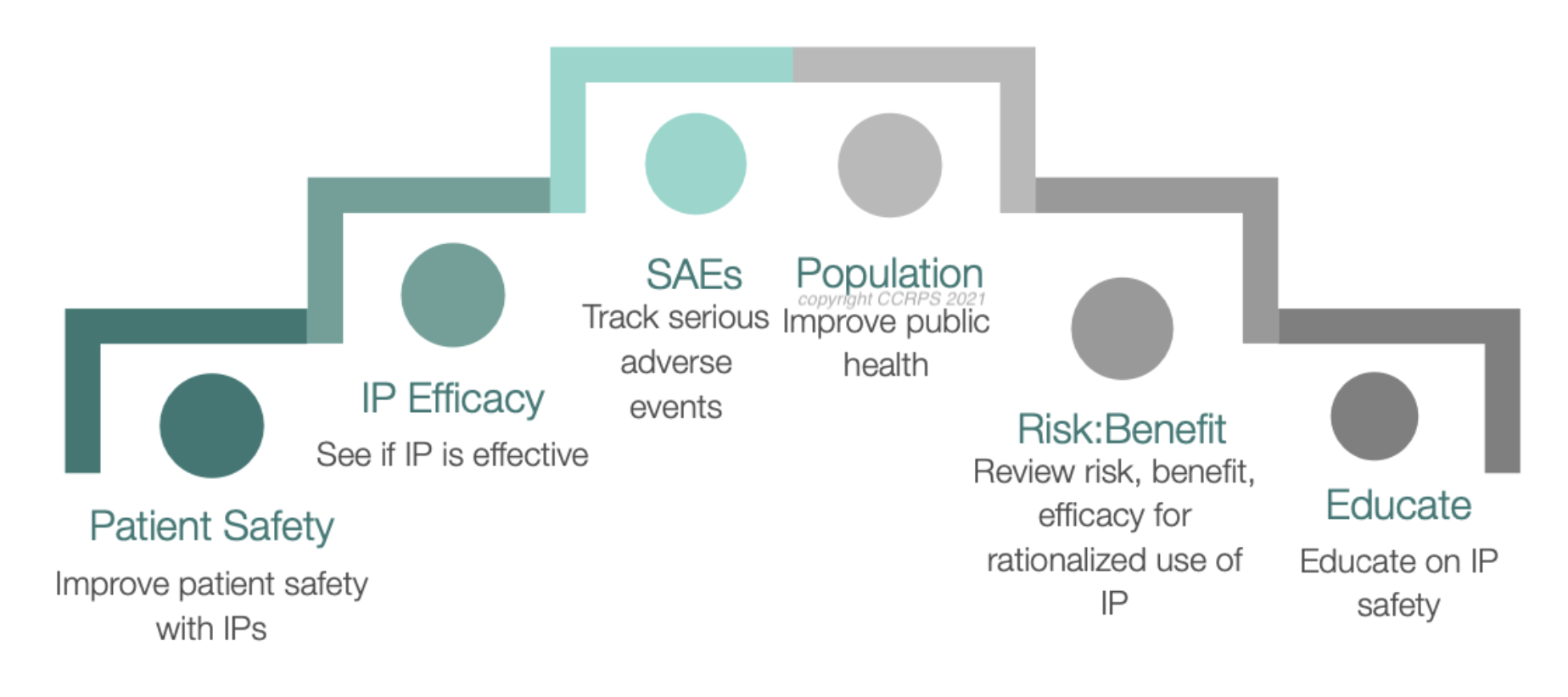
In clinical research, PV operations work with the clinical research team to collect information on a drug’s SAE and ADR. SAE is a serious adverse event. They can be lethal or have major repercussions such as causing disabilities in the patient or inducing birth defects. On the other hand, ADR is an adverse drug reaction. ADRs include a drug’s milder side effects, like headaches, nausea, or fatigue. The safety data collected by the operations division is key to determining a drug’s use and safety.
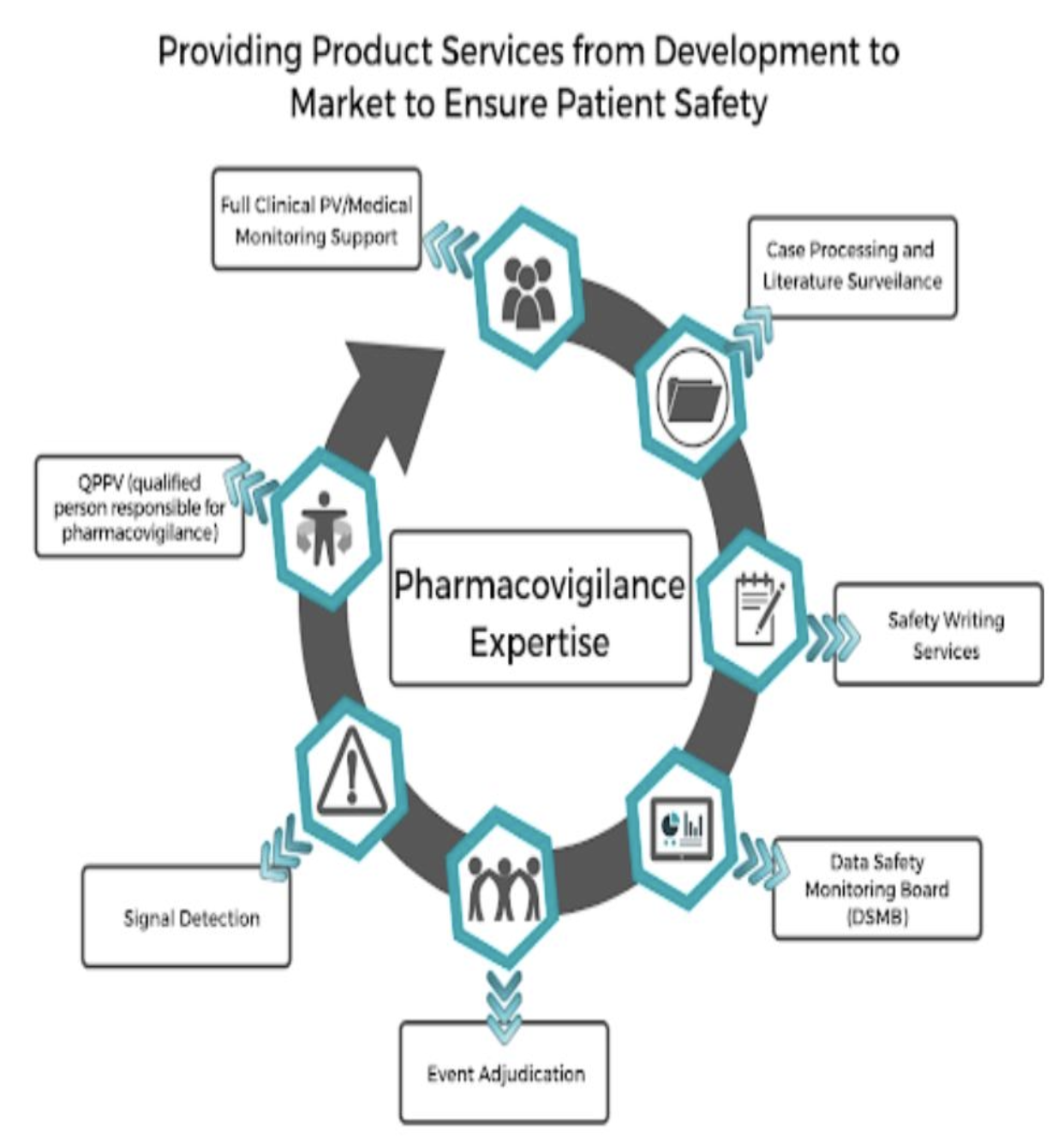
In addition to data collection, the operations team is responsible for creating standard operating procedures (SOPs), as well as individual case study reports, literature screening, and regulatory expedited reporting on Suspected unexpected serious adverse reactions (SUSARs), or unknown as unexpected serious adverse reactions, after the drug is on the market.
The safety data collected by the operations division is maintained by the PV systems division. They ensure that an immense amount of data is organized and accessible to research collaborators. The systems team is always working to improve and maintain safety data, since field regulations and industry expectations are always changing.
After being organized by the systems division, the safety data is then handed to the PV surveillance team. PV surveillance analyzes the safety data and then compiles their insights into development safety update reports (DSURs). This report determines whether or not the drug is safe enough to move on to the next phase of clinical trials. At the end of the third phase, if the drug is safe enough, the PV will submit the drug for FDA approval.
How Do I Start?
The PV department needs and recruits diverse talents to ensure that a drug is safe. If you can see yourself in one of these positions, then you should absolutely consider a career in PV. CCRPS recently launched our pharmacovigilance course to help aspiring professionals learn about the field and stand out in front of employers. If you want to learn more about pharmacovigilance, please visit our website at CCRPS.Org.
For those who are considering further specialization within clinical research, opportunities such as becoming a Clinical Research Coordinator, CRA, or a Clinical Trials Assistant are also available. These roles are critical in the management and execution of clinical trials and offer a pathway to advanced positions like Advanced Clinical Research Project Manager Certification or an Advanced Principal Investigator Physician Certification.
For medical professionals looking to expand their expertise in overseeing clinical trial safety and compliance, our ICH-GCP course and Medical Monitor Certification provide comprehensive training in Good Clinical Practice and the responsibilities of a medical monitor in clinical research.