Clinical Research Certification Kansas: Everything You Need to Know for 2025–2026
Kansas is quietly becoming a Midwest engine for compliant clinical trials, with growth centered around Kansas City, Wichita, and Overland Park. Hospitals, CROs, and university sites increasingly prefer credentialed professionals who can execute ICH-GCP, eTMF, and risk-based monitoring from day one. This guide delivers pure, practical value on certification pathways, salaries, hiring trends, and role progression—anchored to CCRPS programs that align with sponsor expectations and global standards.
1. Clinical Research in Kansas: 2025–2026 Outlook
Kansas’s clinical pipeline is expanding across oncology, cardiology, and immunology, driven by health systems that demand audit-ready documentation and GCP-trained staff. Candidates who complete CCRPS’s ICH-GCP earn instant credibility for site start-up and monitoring. Start with ICH-GCP online and stack a role-specific track like CRA or Clinical Project Management to match hiring plans.
Kansas candidates benchmarking requirements often compare state resources such as North Carolina, Ohio, and New York to align skills with multi-state CRO needs. If you’re targeting remote monitoring, CRA in Oregon and CRA in Pennsylvania guides clarify expectations for decentralized roles.
For entry pathways and lateral moves, many professionals review adjacent state frameworks—Nebraska, Oklahoma, Oregon—to ensure their CV language and competencies map 1:1 with sponsor SOPs.
Kansas Clinical Research Certification Outlook (2025–2026)
| Key Metric | 2025–2026 Data for Kansas |
|---|---|
| Average CRA Salary (KS) | $88,000–$103,000 |
| CPM Salary Band | $110,000–$126,000 |
| Regulatory Specialist | $80,000–$97,000 |
| Projected Job Growth | 16–18% (2025–2026) |
| Top Hiring Cities | Kansas City, Wichita, Overland Park |
| Leading Institutions | Academic Med Centers & Regional Hospitals |
| Major CRO Presence | IQVIA, ICON, PPD, Medpace |
| Entry-Level Roles | CRC, Project Assistant, In-House CRA |
| Preferred Credentials | CCRPS Clinical Research, ICH-GCP, CRA |
| Program Duration | 4–6 weeks (self-paced) |
| Typical Program Cost | $350–$500 |
| Work Model | Hybrid + remote monitoring roles |
| Top Skills in Demand | SAE reporting, eTMF, source verification |
| RBM Adoption | High (KPIs + centralized review) |
| Promotion Velocity | 6–12 months post-certification |
| Internship Pay | $19–$26/hour |
| Therapeutic Hotspots | Oncology, NASH, Cardiology |
| Kansas Hiring Edge | GCP + monitoring readiness |
| Certification Renewal Cycle | Every 2 years |
| Alumni Perk | CCRPS lifetime updates |
| Sponsor Expectations | Protocol adherence, clean queries |
| Site Types | Academic, community, private practice |
| Decentralized Trials | Growing (eConsent, eSource) |
| Data Tools | EDC/eCRF, CTMS, eTMF |
| Competitive Advantage | CCRPS multi-badge stack (GCP+CRA) |
| Grant Landscape | NIH + regional foundations |
| Employer Sponsorship | Available in hospital networks |
| Hiring Signal | Verifiable CCRPS credential |
2. How to Get Certified in Kansas
Kansas employers increasingly screen for GCP mastery, site PM skills, and monitoring fundamentals. The fastest path: complete CCRPS Clinical Research Certification (core operations), add ICH-GCP (compliance), then layer Clinical Project Management or CRA depending on your target role.
If you’re transitioning from bedside or lab roles, review regional playbooks like Mississippi and Montana for skill translation. For international mobility or remote work, leverage ICH-GCP in India plus CRA in North Dakota to mirror multi-jurisdiction standards.
Execution roadmap: finish 50–60 hours of CCRPS modules; pass the exam with a verifiable digital badge; update your CV with GCP, monitoring, SAE reporting, and eTMF bullets. Use CRA in Rhode Island and South Dakota guides to copy recruiter-ready phrasing.
3. Salary and Market Growth in Kansas
Kansas compensation now outperforms uncertified peers by 15–20%, particularly where CROs centralize monitoring. Typical ranges: CRA $88k–$103k, CPM $110k–$126k, Regulatory $80k–$97k. Upside grows with stacked badges (GCP+CRA+CPM) from CCRPS.
If you’re designing your raise path, pair Clinical Project Management (Europe) with CRA (Oregon) to signal end-to-end ownership. For hybrid/remote roles, recruiters frequently cite CRA (South Carolina) and CRA (Pennsylvania) benchmarks.
Compare Kansas with Nevada and New Jersey to calibrate expectations on sponsor saturation and therapeutic diversity; then tune your skills toward the oncology + cardiology mix most common in Kansas postings.
Poll: What’s Your #1 Roadblock to Kansas Clinical Certification?
4. Why CCRPS Is the Preferred Certification Provider
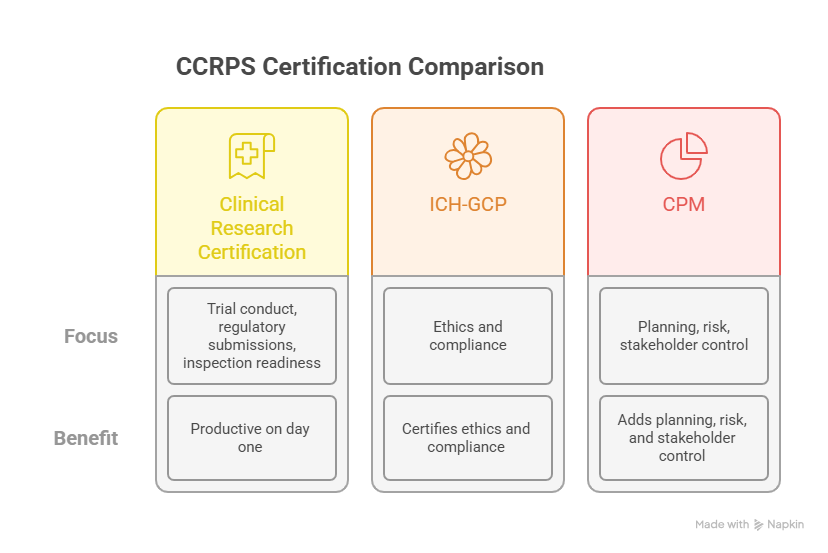
CCRPS is built around sponsor SOPs and CRO expectations, so graduates are productive on day one. The Clinical Research Certification covers trial conduct, regulatory submissions, and inspection readiness; ICH-GCP certifies ethics and compliance; CPM adds planning, risk, and stakeholder control.
For Kansas applicants exploring multi-state careers, review North Dakota and Oklahoma to mirror language that resonates with different IRB ecosystems. If you’re targeting a CRA ladder, compare CRA in South Dakota and CRA in Pennsylvania for KPI expectations (visit load, SDV rate, CAPA rigor).
CCRPS’s lifetime updates keep your SOP vocabulary current, while digital badges streamline HR verification. Stack your profile with GCP + CRA + CPM and recruiters will instantly understand your compliance, monitoring, and leadership capacity.
5. Career Opportunities After Certification
With certification in hand, Kansas candidates can step into CRC, In-House CRA, CRA, Regulatory Specialist, and Project Coordinator roles, then accelerate toward Lead CRA or CPM. Hiring teams consistently shortlist applicants showing GCP badge + role-specific proof from CRA tracks and CPM coursework.
If your goal is remote or regional monitoring, benchmark deliverables against CRA in New York and CRA in North Carolina—mirror their “risk-based plan + deviation analysis + query resolution” language. For market breadth, study Clinical Research Certification in Oregon and New Mexico to understand how decentralized workflows affect workload and KPIs.
Senior tracks open quickly when you combine monitoring metrics (SDV %, query aging) with CPM capabilities (budget, timeline, vendor). Candidates who follow the CRA Rhode Island and South Carolina playbooks routinely secure lead assignments faster.
6. FAQs: Clinical Research Certification in Kansas (2025–2026)
-
A bachelor’s in life sciences, nursing, or pharmacy is typical, but CCRPS accepts relevant clinical or research experience. Map your background using Mississippi and Montana guides to frame transferable skills.
-
Most finish in 4–6 weeks. Combine ICH-GCP online with CPM (UK/Canada) for leadership readiness.
-
Yes—CROs, hospitals, and academic sites value verifiable badges. Compare employer expectations in Nevada and New Jersey to prepare cross-market CVs.
-
CCRPS is fully online and modular. Borrow pacing tips from New Mexico CRA and North Carolina CRA guides.
-
-
Yes—many site networks post externships suited to GCP-ready learners. Review South Carolina CRA for early-role deliverables.
-
CCRPS aligns to ICH-GCP, enabling U.S., Canada, EU, and India mobility. Validate portability via ICH-GCP India and Europe CPM.
-
CCRPS blends operations + compliance + leadership, grounded in sponsor SOPs. For CRA-track clarity, compare Pennsylvania and Rhode Island task lists.