Top 7 Words Every Pharmacovigilance Professional Should Know
Ever wondered what secret language pharmacovigilance pros whisper in the corridors of Big Pharma? Buckle up, word nerds! We’re diving deep into the jargon jungle of drug safety. Here are the top 7 buzzwords you need to sling around like a pro to keep up with the cool kids in clinical research.
7 Key Terms You Need to Know
The "Essential Pharmacovigilance Lexicon" is a vital toolkit for professionals navigating the complex landscape of drug safety. Here’s a thorough breakdown of the key terms every pharmacovigilance (PV) professional should be well-versed in:
1. Adverse Drug Reaction (ADR)
An Adverse Drug Reaction (ADR) refers to any unintended and negative response to a drug, which occurs at any dose and may not be related to the intended medicinal effects of the drug. This term encompasses all types of reactions from the minor, such as mild headaches, to the severe, like a life-threatening rash. In PV, these reactions are meticulously recorded and monitored. The purpose is to ensure that these reactions are understood and, if possible, prevented in future cases. Each ADR is a critical piece of data that contributes to the overall safety profile of a medication.
2. Side Effect
Side effects are known, anticipated reactions to a drug that occur at normal dosages. These effects are generally documented in the drug’s literature and are considered an acceptable risk associated with taking the medication. Unlike ADRs, side effects are expected and often mild, making them less alarming but still noteworthy. They are not the main focus but are monitored to ensure they remain consistent with expectations and do not worsen.
3. Adverse Drug Event (ADE)
An Adverse Drug Event (ADE) broadens the scope beyond reactions specifically caused by the drug to include any unfavorable medical occurrence that happens during treatment with a drug, whether or not directly linked to the drug itself. This term is crucial because it captures all possible negative outcomes during drug therapy, not just those with a clear causal link to the drug. ADEs require careful investigation to determine whether they are indeed caused by the drug or are coincidental.
4. Serious Adverse Event (SAE)
A Serious Adverse Event (SAE) is a significant medical occurrence that has profound health implications. These events include outcomes that result in death, are life-threatening, require hospitalization, cause disability or permanent damage, or involve a congenital anomaly/birth defect. The critical nature of SAEs means that they are a priority within pharmacovigilance practices, with stringent reporting requirements to ensure rapid response and mitigation strategies.
5. Unexpected Adverse Drug Reaction (USADR)
A Unexpected Adverse Drug Reaction (USADR) refers to reactions that are not previously identified or documented in the drug’s labeling. Discovering a USADR is akin to finding an unexpected plot twist—it’s unanticipated and could potentially have serious implications. If the reaction is severe, it is categorized further as a Suspected Unexpected Serious Adverse Reaction (SUSAR), which mandates immediate reporting to regulatory authorities to reassess the drug’s safety profile.
6. Signal Detection
Signal Detection is a critical function in pharmacovigilance that involves analyzing data to identify new or known risks associated with drugs. This process utilizes advanced statistical and data mining techniques to sift through large volumes of data to detect patterns or signals indicative of potential drug effects. These signals could reveal beneficial or adverse effects not previously recognized, prompting further investigation and potentially leading to changes in clinical practice or regulatory policy.
7. Causality Assessment
Causality Assessment is the process of evaluating the relationship between a drug and an adverse event. It is a detective-like investigation where PV professionals determine whether the drug is likely to have caused the adverse event. Assessments range from "Certain" (definite causality) to "Unlikely" (little or no causality), helping to clarify the implications for drug safety and guide regulatory and clinical decisions.
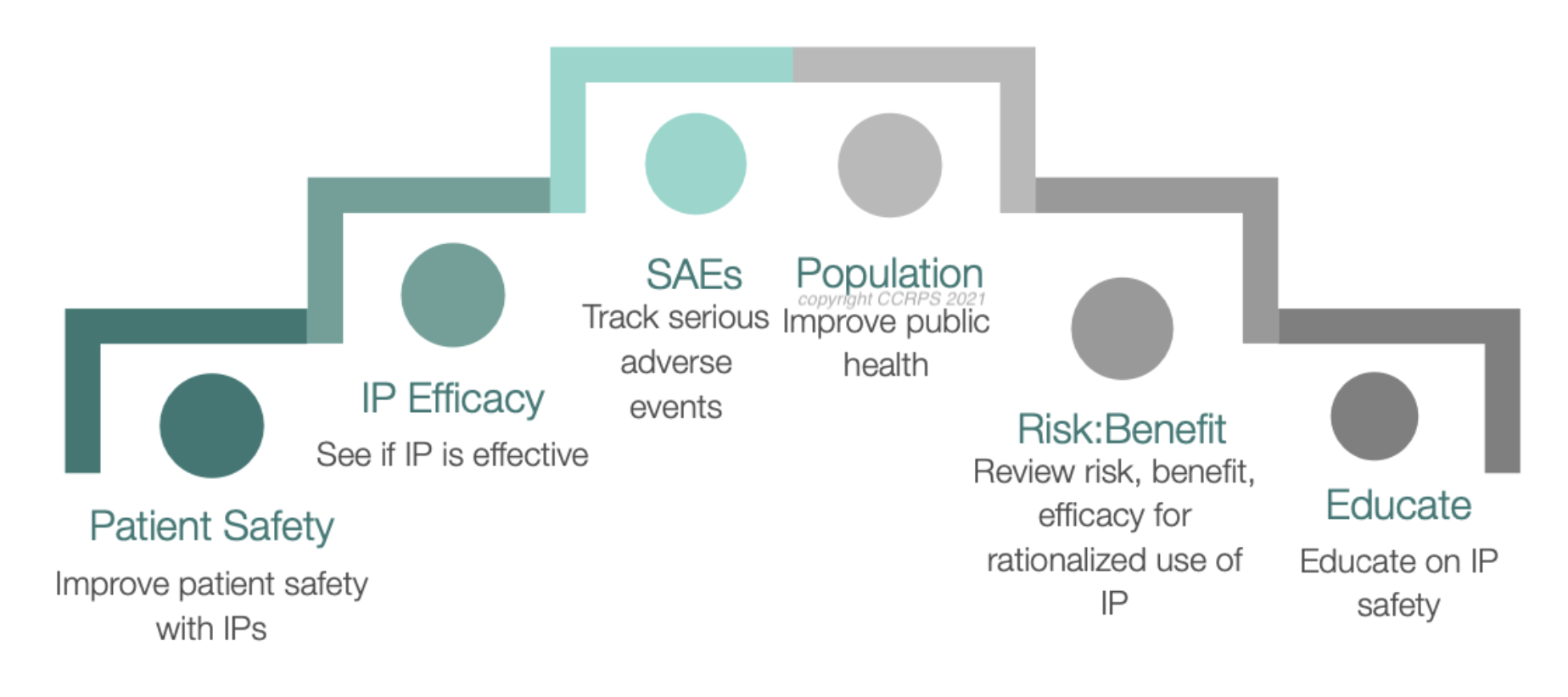
Each of these terms forms the backbone of pharmacovigilance and is essential for ensuring the safety and efficacy of drugs in the market. By mastering this lexicon, PV professionals can more effectively contribute to the protection of public health and the optimization of therapeutic strategies.
Uncommon Facts & Data in Pharmacovigilance
Over 60% of ADRs are preventable. (Source)
The first pharmacovigilance systems were developed in response to the thalidomide tragedy in the 1960s. (Source)
Advanced algorithms now predict potential ADRs based on chemical structures. (Source)
The global pharmacovigilance market is expected to reach $10 billion by 2025.
Approximately 5% of hospital admissions in the U.S. are due to ADRs.
Less than 10% of ADRs are ever reported to national monitoring centers.
The oldest known ADR was recorded on papyrus in ancient Egypt.
Real-world evidence is becoming crucial in understanding long-term effects of drugs.
Digital health technologies are set to revolutionize how ADRs are monitored.
The EU’s pharmacovigilance legislation is one of the most comprehensive worldwide.
Conclusion
For those looking to make a mark in clinical research, mastering these terms and diving deeper through CCRPS's specialized courses could be your best next move. Unlock your potential today!
This rewritten blog aims to be informative, engaging, and comprehensive, equipped to rank highly in SEO for its targeted keywords and answering the nuanced needs of pharmacovigilance professionals in 2025.
Explore Courses for Clinical Research Career
Courses Available:
Frequently Asked Questions (FAQs)
-
An Adverse Drug Reaction (ADR) is any unintended and negative response to a medication, which can occur at any dosage and may not be related to the intended use of the drug. These reactions can be serious or mild and are often unpredictable. In contrast, a side effect is a known and expected reaction to a medication, occurring at therapeutic dosages and typically listed on the drug’s leaflet. While side effects are generally anticipated and less severe, ADRs can range widely in severity and may not always be anticipated.
-
Signal detection in pharmacovigilance involves the identification of unusual patterns or clusters of events that are reported less frequently. Pharmacovigilance professionals utilize statistical tools and data mining software to analyze vast amounts of data from various sources such as clinical trials, patient reports, and literature. The aim is to detect any signal that might indicate a new or previously known risk associated with a drug, ensuring prompt investigation and regulatory action if needed.
-
An adverse drug event (ADE) becomes serious when it results in significant patient harm, including death, life-threatening situations, hospitalization, disability, or permanent damage. Other criteria that categorize an ADE as serious include congenital anomalies or requiring intervention to prevent permanent impairment. The seriousness of an ADE is a key factor in determining the intensity of the response and the need for regulatory reporting.
-
Causality assessment is the process used to determine the likelihood that a drug caused a specific adverse event. This involves:
Collecting detailed patient data: Includes medication history, the timing of the event relative to drug administration, and any other relevant medical information.
Evaluating the event: Comparing the event against the drug’s known side effects and interactions.
Ruling out alternative causes: Assessing other possible factors that could have caused the event.
Re-challenge (if applicable): In some cases, re-administering the drug to see if the event reoccurs, which can strongly suggest causality.
-
Pharmacovigilance is crucial in clinical research as it ensures the safety and efficacy of pharmaceuticals across their lifecycle. By monitoring and evaluating the adverse effects of drugs, pharmacovigilance helps to protect patients from undue harm and supports the development of safer, more effective medications. This ongoing surveillance also informs necessary updates to drug regulations and prescribing guidelines, ultimately guiding clinical practice.