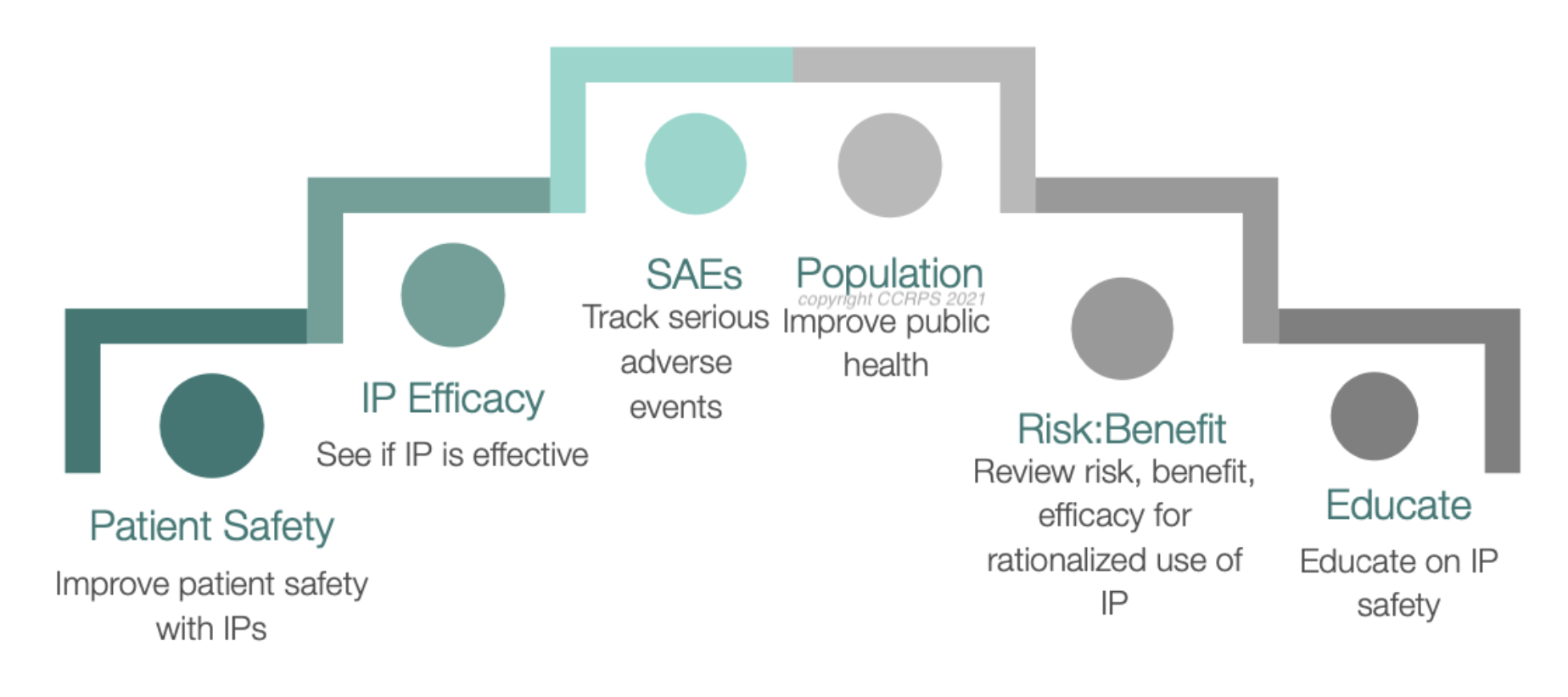
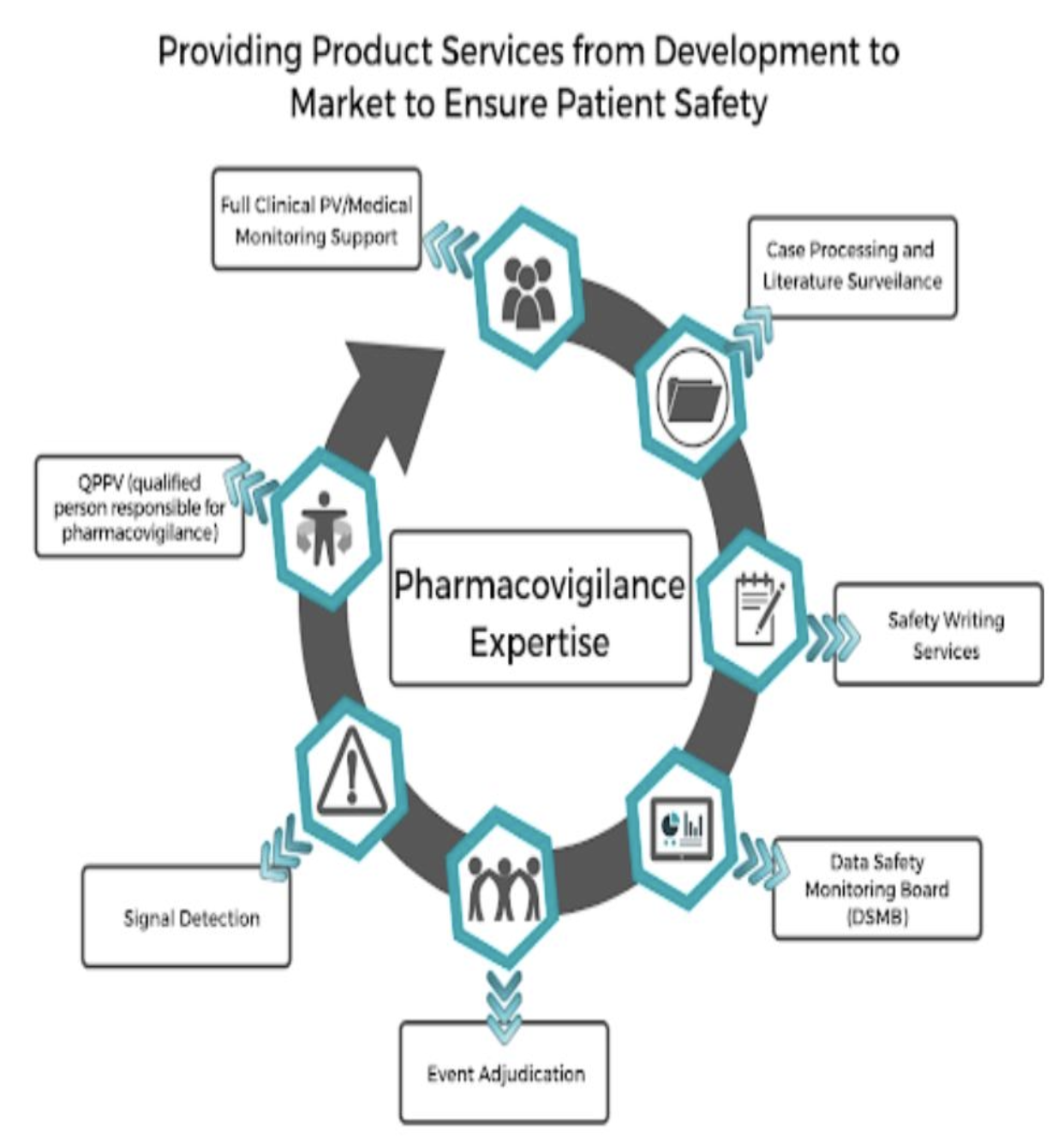
What is Pharmacovigilance (PV)
Pharmacovigilance (PV) is the science and activities relating to the detection, assessment, understanding, and prevention of adverse effects or any other drug-related problems. This critical field of healthcare ensures that the medicines we use are safe and effective, continuously monitoring their performance after they reach the market. Imagine the global healthcare system as a vast ocean, with PV professionals serving as the lifeguards who keep swimmers safe from hidden dangers. These dedicated individuals use their sharp eyes and quick reflexes to spot and manage risks, ensuring that the benefits of medications outweigh their risks.
The Role of Pharmacovigilance
The role of pharmacovigilance (PV) is pivotal in ensuring the safety and efficacy of drugs both before and after they reach the market. This discipline focuses primarily on the detection, assessment, and prevention of adverse drug reactions (ADRs), which are any unintended or harmful responses to a medication when it is taken in normal doses. Here’s a detailed look at how PV protects patients and fosters public health:
Protecting Patients
Such pharmacovigilance systems are meant to ensure that patients are protected by ensuring that the benefits of a drug derived from a product do not have more risks. Such protection is very important not only during the early trials of the drug but during the entire period of use of the drug in the general population. PV experts assess and analyze information from different sources, including clinical practices, practices of healthcare givers, and patients to recognize possible ADRs. They are therefore able to put in place safety measures, change the dosage recommendations or even take drugs off the market where necessary.
Fostering Public Health
Beyond the patient, PV is important to public health. In this way, pharmacovigilance contributes to the continued public confidence in the healthcare system and the pharmaceutical industry by keeping a strict watch on drug effects and adverse drug reactions. Without the ability to store not only the treatment capabilities of drugs but the knowledge that these treatments are safe and monitored, public health depends on it. The trust is especially important in public health emergencies when new treatments or vaccines are being pushed out.
Early Detection of Adverse Drug Reactions
One of the most important roles of PV is the early identification of ADRs. It is not only a matter of gathering reports but also of going in search of possible safety signals before they become a widespread problem. These technologies have improved the capacity to find patterns that might not be obvious through conventional analysis methods, such as data mining and machine learning. Timely interventions are possible because of early detection; these can range from warning healthcare professionals and patients to warning them, to changing regulations.
A Day in the Life of a Pharmacovigilance Professional
A day-to-day work of a PV professional is quite diverse and can be quite demanding as well as satisfying. It’s not always bending over backwards for patients, either; their work varies from reviewing case reports of ADRs to searching for patterns in the data that may be a sign of a safety issue. These professionals are the unsung heroes at the office, whose hard work ensures that the medications that we rely on are safe even after they are approved.
Core Components of a Pharmacovigilance System
The core components of a Pharmacovigilance (PV) system are meant to contribute to drug safety through a systematic method of sensing, reporting, and following up on information on adverse drug events (ADEs) and other issues associated with drugs. This system is an essential part of guaranteeing the safety and effectiveness of pharmaceutical products throughout their product development process. Here’s a detailed breakdown of the essential elements within a pharmacovigilance system:
1. Robust Data Collection
The foundation of any effective pharmacovigilance system is the ability to collect comprehensive data. This data comes from a variety of sources, including:
Clinical Trials: Data from initial and ongoing clinical trials provides initial insights into potential ADRs under controlled conditions.
Healthcare Providers: Reports from doctors, nurses, and other healthcare professionals who observe effects in patients post-marketing.
Patients: Direct reports from patients or caregivers, often collected via patient support programs or through digital health platforms.
Literature and Research Studies: Published medical research and case reports that may reveal new information about drug safety.
Regulatory Authorities: Global databases maintained by authorities like the FDA and EMA, which compile data submitted by pharmaceutical companies and healthcare providers.
The process of data collection must be proactive and systematic to ensure that all potential safety signals are captured and nothing significant is overlooked.
2. Meticulous Documentation
Once data is collected, it must be meticulously documented. This documentation process involves:
Recording Detailed Information: Each report of an ADR or medication error must be recorded with as much detail as possible, including patient demographics, drug dosage, duration of drug use, and a description of the adverse reaction.
Standardization: Using standardized terminology, such as that provided by the Medical Dictionary for Regulatory Activities (MedDRA), to ensure consistency and reliability across different reports and studies.
This detailed and standardized documentation allows for more accurate analysis and helps in identifying patterns that might require further investigation.
3. Comprehensive Data Management
Effective data management is critical in pharmacovigilance. It involves:
Data Integration: Combining data from various sources into a centralized database to facilitate comprehensive analysis.
Data Security: Ensuring that patient and proprietary information is protected according to legal standards.
Data Accessibility: Making data available in a usable format for those involved in drug safety assessments.
4. Risk Assessment
Risk assessment is perhaps the most critical component of a pharmacovigilance system. It involves:
Analysis of Data: Using statistical and analytical tools to identify trends, correlations, and potential safety signals from the vast amounts of data collected.
Evaluation of Risk: Assessing whether identified risks are significant and determining the seriousness and likelihood of these risks.
Benefit-Risk Balance: Evaluating whether the benefits of a drug outweigh its risks, which is a dynamic and ongoing process.
5. Risk Management
Based on the outcomes of risk assessments, risk management strategies are developed. These may include:
Regulatory Actions: Such as updating the drug’s labeling, restricting its use, or even withdrawing the drug from the market.
Communication Strategies: Informing healthcare providers and the public about new risks and how to manage them.
Monitoring Effectiveness: Continuously monitoring the effectiveness of implemented strategies and making adjustments as necessary.
Technologies Shaping the Future of Pharmacovigilance
Advancements in technology, particularly in artificial intelligence (AI) and big data analytics, are revolutionizing pharmacovigilance. AI algorithms can predict potential adverse reactions before they become widespread issues, offering a proactive approach to drug safety. Additionally, big data allows for the aggregation and analysis of vast amounts of health data, enhancing the detection and monitoring capabilities of PV systems.
Navigating Global Pharmacovigilance Regulations
The global landscape of pharmacovigilance regulations is complex and ever-changing. Professionals must stay informed about international regulations and guidelines to ensure compliance and protect patient safety. For example, the European Medicines Agency (EMA) and the U.S. Food and Drug Administration (FDA) have specific requirements for the monitoring and reporting of ADRs, which must be meticulously followed.
Pharmacovigilance Careers: Opportunities and Pathways
Careers in pharmacovigilance (PV) encompass a range of roles that are crucial for ensuring the safety and efficacy of pharmaceutical products. These careers provide professionals with the opportunity to impact public health positively by preventing adverse drug reactions and enhancing medication safety. Here’s a deeper exploration of the opportunities and pathways available in pharmacovigilance:
1. Diversity of Roles
Pharmacovigilance offers a variety of roles that cater to different interests and skill sets within the healthcare and pharmaceutical sectors. Some of the key positions include:
Pharmacovigilance Officer/Specialist: Responsible for the day-to-day activities related to the detection, assessment, understanding, and prevention of adverse effects or any other possible drug-related problems.
Pharmacovigilance Scientist/Analyst: Focuses on analyzing data from clinical trials and post-marketing sources to identify potential safety concerns.
Risk Management Specialist: Develops strategies to minimize risks associated with drug use and ensures compliance with regulatory requirements.
Regulatory Affairs Manager: Works on the submission of drug safety reports to regulatory agencies and ensures compliance with regulations in different countries.
Clinical Data Manager: Manages and analyzes clinical trial data to ensure that it meets the necessary standards for accuracy and reliability in assessing drug safety.
2. Sectors of Employment
Professionals in pharmacovigilance can find employment in various sectors, each offering unique experiences and challenges:
Pharmaceutical Companies: Many pharmacovigilance professionals work in the pharmaceutical industry where they manage clinical trial data, report on adverse drug reactions, and ensure compliance with global drug safety regulations.
Biotechnology Firms: In smaller, more research-oriented companies, PV professionals may have a broader range of responsibilities, including overseeing entire safety and regulatory processes.
Contract Research Organizations (CROs): These organizations provide support to the pharmaceutical and biotech industries in the form of research services outsourced on a contract basis, including drug safety monitoring.
Government and Regulatory Bodies: Such as the Food and Drug Administration (FDA) or the European Medicines Agency (EMA), where professionals help to formulate safety regulations and monitor compliance across the industry.
Academic and Research Institutions: Engaging in research related to drug safety and pharmacovigilance systems, contributing to the academic and practical knowledge base.
3. Required Education and Skills
A career in pharmacovigilance typically requires:
Educational Background: A bachelor’s degree in pharmacy, nursing, biology, chemistry, or a related field is usually necessary. Advanced degrees (e.g., PharmD, PhD, or MD) can enhance prospects, especially for higher-level positions.
Relevant Skills: Strong analytical skills are essential for assessing data related to drug safety. Communication skills are also crucial, as professionals must often explain complex information to regulatory authorities, healthcare providers, and the public.
Certifications and Training: Specific pharmacovigilance training programs or certifications, such as those offered by the Drug Information Association (DIA) or other professional bodies, can provide an advantage.
4. Career Advancement
Career advancement in pharmacovigilance often involves gaining experience in various aspects of drug safety, completing advanced education, and obtaining certifications. Professionals may start as junior analysts or associates and can move up to senior roles such as department heads or directors of pharmacovigilance. Leadership roles typically require a deep understanding of both the scientific and regulatory aspects of drug safety, as well as strong management skills.
Innovative Practices in Pharmacovigilance
In the realm of pharmacovigilance (PV), innovation is pivotal in evolving the methods and technologies used to ensure drug safety. The incorporation of new practices such as wearable technology and advanced data analytics are revolutionizing the field, enhancing both the efficiency and effectiveness of monitoring drug effects. Here’s a detailed exploration of some of these innovative practices:
Wearable Technology
One of the most exciting advancements in pharmacovigilance is the integration of wearable technology to monitor drug effects in real-time. Wearable devices, such as smartwatches and fitness trackers, can collect a vast array of health data from patients, including heart rate, blood pressure, temperature, and even electrocardiogram readings. This data can provide immediate, continuous feedback on a patient’s response to a medication, offering several benefits:
Timely Detection of Adverse Drug Reactions: Wearables can alert both patients and healthcare providers to potential adverse reactions much faster than traditional methods, which often rely on patient reports during follow-up visits.
Personalized Medicine: Real-time data helps in tailoring drug doses to individual needs, potentially reducing side effects and improving drug efficacy.
Increased Patient Engagement: By involving patients directly in monitoring their health, wearables increase patient engagement and adherence to treatment protocols.
Big Data and Advanced Analytics
The vast amounts of data generated by wearable technology and other digital health tools can be overwhelming. However, with the advent of big data technologies and advanced analytics, pharmacovigilance professionals can harness this data to gain profound insights into drug safety:
Data Mining: Sophisticated algorithms can sift through large datasets to identify patterns that may indicate potential adverse effects or interactions with other medications.
Predictive Analytics: These tools can forecast individual risks by analyzing past adverse reactions and patient outcomes, potentially preventing future incidents.
Machine Learning: AI and machine learning models can continuously learn from new data, improving their predictive accuracy over time and helping regulators and healthcare providers make informed decisions about drug safety.
Digital Health Records and Integration
Integrating digital health records into pharmacovigilance systems is another innovative practice. This integration allows for seamless access to a patient’s medical history, medication list, and other health information, which can be critical for accurately assessing drug safety:
Holistic View of Patient Health: A more comprehensive view helps in understanding the context of adverse reactions, such as pre-existing conditions or concurrent medications that may influence drug effects.
Streamlined Reporting: Automation of data collection from electronic health records (EHRs) can streamline the reporting process, making it easier and faster for healthcare providers to submit accurate and complete safety reports.
Social Media and Real-Time Monitoring
Social media platforms and online forums are increasingly being used as tools for real-time monitoring of drug effects. Patients often discuss their experiences with medications on these platforms, providing a rich source of real-world evidence:
Patient-Reported Outcomes: Analysis of social media can uncover patient-reported outcomes and adverse effects that might not be reported through traditional channels.
Sentiment Analysis: This technique analyzes the sentiment and tone of online discussions to gauge public perception and acceptance of medications, potentially identifying issues early on.
Explore Career Advancements in Pharmacovigilance
Pharmacovigilance Certification
This certification will equip you with the necessary skills to effectively manage and report adverse drug reactions and ensure compliance with regulatory laws.Clinical Research Coordinator Certification
Learn the fundamentals of clinical research coordination, enhancing your ability to manage clinical trials and patient care effectively.CRA Certification
As a Clinical Research Associate, gain expertise in monitoring clinical trials and ensuring adherence to established guidelines and regulations.ICH-GCP Certification
This certification provides thorough training in the International Conference on Harmonisation - Good Clinical Practice guidelines, crucial for anyone involved in clinical trials.Clinical Trials Assistant Training
Prepare for a role as a Clinical Trials Assistant, where you will support the administration and operational aspects of clinical trials.Advanced Clinical Research Project Manager Certification
Develop advanced skills in managing complex research projects, overseeing trial progress, and ensuring projects meet their objectives.Advanced Principal Investigator Physician Certification
This course is designed for physicians who wish to lead clinical trials, focusing on the intricacies of trial management and regulatory compliance.Medical Monitor Certification
Gain specialized skills in monitoring and overseeing the medical aspects of clinical trials, ensuring patient safety and the integrity of clinical data.
Conclusion
As we've explored the critical world of pharmacovigilance, it's clear that the field is more than just a technical necessity; it's a dynamic area of clinical research dedicated to enhancing patient safety and therapeutic outcomes. For those looking to enter or advance in this field, CCRPS offers a pharmacovigilance certification course designed to equip you with the knowledge and skills needed to make a difference. Dive into the world of PV with CCRPS and become part of the next generation of drug safety experts.
Frequently Asked Questions (FAQs)
-
A bachelor's degree in health sciences, pharmacy, nursing, or medicine is typically required, along with relevant experience in clinical settings.
-
By ensuring drug safety and efficacy, PV protects the public from potential adverse effects and enhances trust in healthcare systems.
-
Challenges include staying updated with regulatory changes, managing large data sets, and ensuring timely response to drug safety issues.
-
Technology, especially AI and big data, has greatly improved the efficiency and effectiveness of adverse event detection and analysis.
-
Data is crucial for identifying safety trends, informing regulatory decisions, and guiding healthcare practices.
-
Yes, healthcare professionals, patients, and caregivers can all report ADRs, which are crucial for pharmacovigilance activities.
-
PV is essential in clinical trials to ensure participant safety, identify ADRs early, and adjust study protocols if necessary.
-
Regulations vary by country but generally require rigorous ADR reporting and monitoring to ensure drug safety and public health.