7 Reasons Why You Should Get into Clinical Research
Have you ever thought that there’s a perfect job out there for you, but you just haven’t found it yet? If you are motivated, informed, and interested in a science and medical career, you might have just found your answer. The future in clinical research is bright, and it is one that you will want to be a part of.
The clinical research industry is a highly lucrative and expanding field. The global clinical trials market has been estimated at $80.7 billion in 2024. As the push for new vaccines and therapeutics climbs, the field value is expected to grow even more. Experts predict that the global market will hit $123.5 Billion By 2030.
Contrary to popular belief, working in clinical research doesn’t have to mean you have to stay in a lab. There are demands and opportunities for every skill set, if you know how to find them. Below, I have put together an in-depth guide on why you should get into clinical research.
You like to a job that’s flexible
Don’t like working in a cubical? How about heading to the airport every morning instead? If you like a job that keeps you moving, then becoming a Clinical Research Associate (CRA) might be the right move for you.
CRAs, contrary to what most believe, don’t collect data or interact with patients. A CRA’s job is to travel between different research sites and verify data transcription. They are called “monitors” because it is their job to ensure that every site is following proper compliance and protocols.
There are two types of CRAs: home base and in-house. Home base CRAs work remotely. That means they work and travel from home. If you get tired of working from home, you can become an in-house CRA. In-house CRAs stay in one site and work together with a home base CRA to keep each other updated with what is happening at their site.
You like working with people
Have you ever been told that you are a people person? If talking to new people everyday sounds like something you’d enjoy, you should definitely look into becoming a Clinical Research Coordinator (CRC).
CRCs are the backbones to every project. They conduct patient visits, input source documents into the electronic data capture (EDC), and ensure that every trial is following compliance. They are also responsible for handling regulatory documents and updating the Principal Investigator (PI) with trial results.
CRCs conduct a variety of tasks, all of which impact the progress and development of the trial. Every successful clinical trials team needs a good CRC. So, if you have strong interpersonal skills and know how to stay organized, you will be indispensable.
You are tech-savvy
Are you a self-proclaimed techie? Perhaps you’ve dabbled in coding, pick up computer programs easily, and maybe even have a background in IT. Technology is the future. If you think you have a knack for organizing data, you should look into becoming a Clinical Trial Assistant (CTA).
CTAs, also known as Clinical Research Assistants, manage the Trial Master File (TMF). They file, archive, and maintain trial documents and study files. They are also responsible for closing inquiries from the CRA, as well as providing administrative support to the research team. Every important step in clinical research, pre-clinical research, study startup, site management, needs a dependable CTA.
While most jobs in clinical research require some understanding of technology, it is especially important for the CTA to know what they are doing when it comes to managing trial documents and study files. In addition, it is equally important that the CTA is organized and pays attention to detail.
You like a good salary with room for promotion
Current Salary Data (USA, 2024):
CTAs:
Average salary: Update to $66,007 (according to Salary.com)
Salary range: Update to $57,991 - $73,867 (according to Salary.com)
CRAs:
The salary progression for CRAs with experience (one to two years - $72,358, seasoned - $110,102) and individual contractor CRAs (up to $300,000) can stay as is.
General salary comparison: The point about the average US base salary being $40,000 and CTA/CRC salaries being good for entry-level positions remains valid.
salaries can vary depending on location, experience, and specific employers.
You don’t want to go back to school
In clinical research, experience is often valued over degrees. Rather what you didn’t study in college, hiring managers are more interested in what you have done in the past and how they can integrate you into their company. While graduate programs can help point you in the right direction, you don’t need a master’s degree to succeed in clinical research. In fact, certain positions don’t even require a bachelor’s or associate’s degree.
Applying to CRC and CTA positions are one of the most common segways into higher positions in clinical research. CRCs don’t need a bachelor’s or associate’s degree to get their foot in the door. While both CTA and CRA positions require a bachelor’s degree, they don’t have to be in the life sciences.
One of the best ways to gain experience and stand out from the crowd is to have on-site experience. If you need advice on how, Dan Sfera, the CEO of DSCS CRO Clinical Research Services, recommends getting started by interning or volunteering at clinics and research sites to build connections and experience. Sometimes, the easiest way to get involved is to offer services like patient recruitment and social media management in exchange for opportunities to build your CV. By appealing to a site’s needs, this will help you get your foot in the door and build the connections and resume you need.
Another great way of adding experience to your resume is by training through certification courses. When employers see that you have taken the time and effort to understand how to be the best in their field, they are more far likely to hire you. At CCRPS.org, we offer seven courses and certification trainings to give you an advantage. 82% of our students are hired within the first month of taking the course. We are accredited by the Accreditation Council For Clinical Research & Education (ACCRE) and tailor our course to you. For example we offer special courses for nurses and an accelerated certification + internship opportunity for anyone with minimal or no clinical experience.
You come from a different field
Switching career fields can be nerve wracking. However, it is also an opportunity for you to be a unique candidate. Whether you come from a closely-related background, like medicine or nursing, or something completely different, there are ways you can advocate for yourself in front of employers.
If you already have a background in medicine, your knowledge of healthcare and your passion for patient health will make for a smooth translation into clinical research. In addition, your RN or MD degrees will help you gain a competitive edge and allow you to climb higher positions, such as the PI, who is the primary researcher of an operation.
On the other hand, if you come from a less relevant field, you can still leverage yourself to be exactly what the clinical research field needs. For example, if you are a teacher, your communication and interpersonal skills will be your keys to success. If you are a lawyer, your ability to draft and read papers will far surpass the average candidate. If you studied mathematics, you are a skilled problem solver. If you are a translator, your language skills are valuable and will help you get into roles that require it. In short, whatever skills helped you succeed in your previous positions, you can bring it with you to clinical research.
You want to make a difference in the world
There are two types of people in the world: ones who accept the world as it is, and ones who strive to change it. In the last 50 years, science and medicine have gone through a series of drastic changes. However, anyone who works behind the scenes will tell you that medical breakthroughs are not miracles. Clinical research is the culmination of human effort and intelligence. The fruits and labor of the ever-expanding industry are proof that if enough people care about the world, then they can change it. While there are many good reasons to work in clinical research, if you want the privilege to enrich the lives of others, there is a place for you in this field.
If you want to take a sneak peak at employers and opportunities near you, jobs sites like Indeed are a great resource.
Here are links for aspiring CRAs, for CRCs, and for CTAs. (Note: CTAs are often referred to Clinical Research Assistants, not to be confused for Clinical Research Associates)
Unlocking Your Future in Clinical Research: Discover the Perfect Career Path for You
Take courses from CCRPS and learn more on how to become a clinical research professional.
Discover more from Clinical Research Training | Certified Clinical Research Professionals Course
CITI Clinical Research Coordinator Course
Significance of CITI Clinical Research Coordinator Course
The diseases that impact the world need solutions. To find aid for the physical and mental issues of this generation, the group of experts in the medical field work day and night. However, they can’t do this on their own. They need clinical research coordinators to help them find success. In this article, you will get to know the significance and responsibilities of clinical research coordinators, and why programs like the CITI Clinical Research Coordinator Course are critical to aspiring professionals and the field as a whole.
Who is a clinical research coordinator?
Basically, a clinical research coordinator works with a principal investigator in medical research. The principal investigator is responsible for large aspects of the project, such as securing grants and writing protocols, while the clinical research coordinator is responsible for the day-to-day operations of research. Some tasks include:
Maintaining records and documents
Recruiting patients
Ensuring trials are following protocol
Keeping the principal investigator informed on developments
Managing supply inventory
The clinical research coordinator needs to complete a variety of tasks, but overall they need to have strong communication and organization skills. For those looking to advance in this role, the Advanced Clinical Research Project Manager Certification might be of interest.
More about the Clinical Research Coordinator Course
Being a clinical research coordinator is not easy. Thus, education and re-education is critical to field professionals.
Many different institutes and universities all around the world offer courses, but the CITI clinical research coordinator course stands out. The course is balanced and well-thought out. Students get to learn about ethics as well as the responsibilities of the clinical coordinator. The institutes do not only teach textbook knowledge, but they also encourage practical knowledge. For a comprehensive learning experience, students might consider exploring related certifications like Pharmacovigilance Certification or Clinical Trials Assistant Training to gain insights into different aspects of clinical trials. Those in more specialized roles may find the Advanced Principal Investigator Physician Certification and Medical Monitor Certification to be valuable additions to their educational pathway.
If you feel inspired, check out their course page here. If you want to compare options, try CCRPS. We offer affordable, ACCRE accredited courses dedicated to clinical research coordinators. Similarly, our courses are written by real professionals that know how to help someone stand out in the field. Still not sure yet? Below are some of our articles that will help you better understand clinical research and make an informed decision.
Take courses from CCRPS and learn more on how to become a clinical research professional.
Discover more from Clinical Research Training | Certified Clinical Research Professionals Course
How to Save Money on Becoming a Clinical Research Coordinator (CRC)
If you think practically, then you will find that nothing in this world comes for free. But if you have a passion for clinical research and need to learn on a budget, here are some ways you can enhance your understanding without breaking the bank.
To understand how you can learn for free, it’s first important to understand the typical trajectory of a clinical research coordinator:
A person seeking clinical research coordinator training should complete high school. There they learn must-have all the science subjects like physics, chemistry, and biology.
After high school, institutes for professional clinical research coordinator professionalism offer programs that are essential for later.
In addition, one can get an experience graduate certificate from an online source. This would help the person reach their career goals faster.
Alternatively, they can complete a bachelor's degree of science.
After completing the bachelor's degree, a master's degree is needed for some of the higher pay-grade positions.
How to get free clinical research coordinator training?
If looking at the education requirements for a clinical research coordinator makes you dizzy, you’re not alone. Becoming a clinical research coordinator takes a lot of time and money. That is why many students turn to scholarships and healthcare programs to help pay their tuition.
On the other hand, there are many websites that offer free or affordable information and training for aspiring professionals. While they can’t replace a formal education, they can supplement your resume and knowledge. While some important topics include clinical data management, pharmacovigilance, and regulatory authorities, you should strive for a comprehensive understanding of the field. At CCRPS, we offer affordable courses designed for clinical research coordinators as well as free ICH GCP training. These can help you build your resume and land the position you want.
In addition, it is really important to keep updated with new clinical research headlines. Below, I have complied some articles that aspiring professionals might find useful. The best thing you can do for your education is to start now and not later.
Take courses from CCRPS and learn more on how to become a clinical research professional.
Discover more from Clinical Research Training | Certified Clinical Research Professionals Course
Why Are So Many Student Taking Clinical Research Courses?
Clinical research courses are becoming a popular subject worldwide. Many students pick this course because of their interest in clinical health science but are drawn to research. The scope of the course is to help students evaluate and understand the new medicines and drugs that are used in clinical trials. This help students find jobs that deal with pharmaceutical products, diagnostic devices, drugs, medicines, and treatment tools.
What is clinical research?
Clinical research can be divided into animal and human trials. These trials help evaluate the effectiveness of drugs and ensure the safety of medical devices. To develop a plan, researchers need to ask questions like:
Which patient who undergo the trial?
When will the trial start?
What kind of treatment should be given?
How can we monitor the processes at every stage?
What is the duration of the whole study?
Researchers involved in these trials often require comprehensive training and certification, such as those offered through a Clinical Research Coordinator course or Pharmacovigilance Certification.
Benefits of clinical research courses
Clinical research helps change and redefine what is possible with medicine. People in clinical research do what they do because they know that their work can change someone’s life.
Clinical research courses have helped many aspiring students become capable professionals. In fact, those who have taken the course often were able to negotiate for more benefits and compensation. Advanced courses like Advanced Clinical Research Project Manager Certification and Advanced Principal Investigator Physician Certification can further enhance career prospects.
How do I find the right course for me?
There are many institutions where one can successfully complete their course. Most courses are offered by health science oriented universities and medical schools. They are generally categorized into three types:
The undergraduate degree is open to students with basic schooling in health sciences. The duration of course study may be 3 to 4 years.
The master’s degree is open to life sciences, medicine, nursing, and pharmacology students. The duration of course study is 2 years.
The doctoral program is open for students with a postgraduate degree in life sciences or clinical research. In most universities, the entire duration of the course is 3 years.
For those looking to specialize further, certifications such as ICH-GCP, CRA, and Medical Monitor Certification provide targeted training for specific roles within clinical research.
When you are looking for a clinical research course, the best thing to do is find the best college. Research colleges based on information about the institution and the types of courses that they offer. You should especially inquire if the institution offers other medical programs. Browse some of our other articles below to get more information on clinical research education.
Remember, even as you become a professional, your education should never stop. CCRPS is here to ensure that you have all the tools you need to succeed. We offer online training courses that are accredited by ACCRE and are tailored to the position you want.
Take courses from CCRPS and learn more on how to become a clinical research professional.
Navigating the World of Clinical Research Education
Courses:
Clinical Research Coordinator Course - Gain the essential skills and knowledge required to coordinate clinical trials effectively. Learn about trial planning, patient selection, treatment administration, and process monitoring.
Pharmacovigilance Certification Program - Explore the critical aspects of drug safety monitoring in clinical research. Understand the regulations, procedures, and reporting systems involved in pharmacovigilance.
Advanced Clinical Research Project Manager Certification - Elevate your career with advanced training in project management specific to clinical research. Learn to lead complex research projects, manage teams, and ensure efficient trial execution.
Advanced Principal Investigator Physician Certification - Develop expertise as a principal investigator physician in clinical research. Acquire advanced knowledge in trial design, patient management, ethical considerations, and regulatory compliance.
ICH-GCP Certification - Obtain certification in Good Clinical Practice (GCP) guidelines established by the International Council for Harmonisation. Understand the ethical and scientific quality standards for conducting clinical trials.
Certified Clinical Research Associate (CRA) Training - Prepare for a career as a clinical research associate responsible for monitoring and managing clinical trials. Learn about protocol compliance, data collection, and regulatory requirements.
Medical Monitor Certification - Specialize in medical monitoring roles within clinical research. Acquire expertise in safety assessment, adverse event reporting, and medical oversight during trials.
What Clinical Research Professionals Need to Know About CRT
Based on your instructions, I've added the relevant course links from CCRPS to the provided blog content. The links are inserted in appropriate sections without altering the original meaning of the content or removing any existing links. Here’s the updated content:
When people are interested in pursuing clinical research, they often need to take clinical research courses to become ready. In clinical research training program courses, students are trained in some common tasks such as clinical research billing, report writing, traits testing, and drug tests. In addition to all this, trainees are given a demo with equipment used in clinical research hospitals. Sometimes, lucky trainees are given a demo on CRT therapies.
CRT is known as cardiac resynchronization therapy. These therapies are done with a CRT pacemaker when patients have heart failure. In general, the CRT device comes in two types: CRT-P and CRT-D. In most cases, trainees can only demo the CRT-P device.
How does the CRT-P device work?
CRT-P consists of two components: the pulse generator and thin insulating wires. This device delivers tiny electrical signals to the left and right side of arteries and ventricles via leads, which makes the heart contract and pump like a normal heartbeat.
CRT-P devices are similar to normal pacemakers, delivering small signals to leads which make the ventricles contract at a normal rate.
The CRT-P device has a battery that is built within the device. When the CRT-P battery runs out, it is necessary to replace the entire device.
The battery limit is determined by a doctor based on what kind of therapy you need.
Although the device is good at providing efficient heart beat rate, when patients are done with the CRT-P pacemaker, there are several drawbacks also. Patients need to have regular checking on batteries of the CRT-P pacemaker device. The doctors would need to check the remaining energy in the device.
Moreover, there are some risks after the implantation of the CRT-P device, such as irritation of the skin all around where the device is placed. There also are several chances for place movements. If you wish to have further updates on clinical research training courses and medical equipment details then you can visit Clinical Research Coordinator courses at CCRPS.
Take courses from CCRPS and learn more about how to become a clinical research professional. You can start with our Pharmacovigilance Certification or explore other opportunities like CRA Certification, ICH-GCP Training, Clinical Trials Assistant Training, Advanced Clinical Research Project Manager Certification, and Advanced Principal Investigator Physician Certification. For those looking into monitoring roles, our Medical Monitor Certification might be the perfect fit.
Discover more from Clinical Research Training | Certified Clinical Research Professionals Course.
Why You Should Take ASH CRTI With Your Clinical Research Course
When it comes to CRTI or clinical research training institute, trainees would learn valuable lessons in topics such as clinical research billing process and traits testing. All these would help them in pursuing clinical research jobs. For those looking to specialize further, the Clinical Research Coordinator course or the Clinical Trials Assistant Training offered by CCRPS can be a great addition to your professional training.
ASH CRTI is the American Society of Hematology, which has developed a clinical research training institute where trainees get experience from senior faculties related to patient oriented clinical research (POCR).
What made ASH CRTI differ from other CRTIs?
ASH clinical research training institute, or ASH CRTI, is a unique training given for people who need to be an early POCR investigator for one whole year. Here, about 20 trainees per year are trained by senior and junior faculty mentors. Here are some of the major components of the ASH clinical research training institute course are listed below:
Workshops will be conducted for a week in California, where trainees will learn about methodologies, foundations and patient-oriented clinical research applications. For those interested in regulatory standards and best practices in clinical research, consider the ICH-GCP course.
After a week of workshops, trainees would follow up for ASH annual meetings where they can listen to experiences from previous scholars. Those aiming for higher responsibility roles might find the Advanced Clinical Research Project Manager Certification useful.
A special one-day class would be conducted at the headquarters of ASH, which is located in Washington, DC. To prepare for such high-level interaction, the Medical Monitor Certification could provide essential insights.
Trainees would be given in-person meetings where they would be taught about networking and mentorship via distance learning. For more comprehensive training in drug safety, the Pharmacovigilance Certification is recommended.
In addition to one-on-one conversations with representatives and training faculties, trainees would have further interactions with their small trainee group along with mentors throughout the year. This interaction would ensure effective research collaborations and career development. Those looking to lead clinical trials might be interested in the Advanced Principal Investigator Physician Certification.
When people take ASH CRTI training along with CRTI course, they have additional resources for success in clinical research. If you wish to get more details about the ASH training course then you can visit ccrps.org.
Take courses from CCRPS and learn more on how to become a clinical research professional. Consider enhancing your career by becoming a Certified Research Associate (CRA).
Discover more from Clinical Research Training | Certified Clinical Research Professionals Course
Clinical Research and Healthcare
Clinical research is part of the health care science that define the care and effectiveness of the medication, it’s devices and diagnostic products and treatment procedures that intended for human use.
Clinical research is a part of healthcare science and it determines how effective and safe diagnostic products, devices, and medications really are. Basically, it studies things that can be used for the diagnosis, treatment, and even prevention of diseases.
Clinical research is very different from other clinical practices. In clinical practice, the focus is on treatments already in use. In clinical research, evidence and innovation is creating new treatments for clinical practice.
The Scope
Clinical research is a broad term that refers to the study and testing of biologics, devices, and drugs. It includes any tests from lab inception all the way to introduction to the market.
First, a molecule or candidate to be tested has to be identified first. This is usually happening in a lab. Pre-clinical studies are then done on the chosen candidate, and then tested on animals. This is where efficacy and toxicity are determined and studied before being tested on humans.
Due to the risks, clinical research has to be authorized by the necessary bodies. A lot of supervision and care has to be taken to ensure that all test subjects are well taken care of and safe. This is because trials need to be conducted on humans, healthy or otherwise, before any drug is approved for market and mass consumption.
Where is it conducted?
In most cases, this kind of research is done at medical centers, especially the academic ones. There are also affiliated study sites that can be used. The sites are situated in areas where there is a high chance of getting many medical participants. There are review boards and regulatory bodies, such as the FDA, that make sure that clinical research is carried out in an ethical manner.
Clinical research involves a very complex network and all have to work for the benefit of everyone. To learn more, check out our website CCRPS.
Take courses from CCRPS and learn more on how to become a clinical research professional.
To learn more about how you can become a clinical research professional, visit our website and explore our diverse range of courses.
Explore our courses: Clinical Research Coordinator
Pharmacovigilance Certification
Clinical Trials Assistant Training
Advanced Clinical Research Project Manager Certification
Clinical Trial Research Aimed at Assessing Behavioral, Surgical, and Medical Intervention
Clinical trials research are studies that are carried out on people. They are usually aimed at the evaluation of behavioral, surgical, and medical intervention. For example, there are different ways that researchers apply clinical research to discover something new about a medical device, diet, or even drugs. Others aim at identifying diseases as early even before any symptoms are evident. They are used to find ways in which health problems can be prevented. Additionally, clinical trials may also be conducted to find ways in which life can be better for persons who are living with diseases that are life-threatening or people with chronic health issues.
What is done before approval?
Before any clinical trial research is approved, laboratory tests have to be conducted by qualified scientists.
Studies are first done on animals subjects. This is done to test the therapy’s potential efficacy and safety. If favorable results are achieved, then the necessary regulatory body can give approval for it to be tested on human subjects.
The phases
The advancement of clinical trials research covers different phases to understand the dosage and identify side effects. When these four phases are complete and the drug or device is seen as favorable, then it receives approval for mass use. However, researchers will continue monitoring the product even after it is introduced in the market.
During phase one, tests are done on healthy people, but in a small group to find the correct dosage, and side effects.
The second phase involves more people and this time the aim is to find the treatment or device’s effectiveness. It offers data on whether a condition or disease can be combated by the drug.
The third phase is about effectiveness and safety. Different dosages and different populations are tested. The drug can be combined with others to see how that impacts the body. This is the point where the product gets FDA approval.
In phase four, after approval, the safety, and effectiveness of the device or drug are tested on a more diverse population for long term effects and monitored after it goes on the market.
Explore our comprehensive range of courses designed to equip you with the knowledge and skills needed to excel in clinical research:
Take courses from CCRPS and learn more on how to become a clinical research professional.
Discover more from Clinical Research Training | Certified Clinical Research Professionals Course
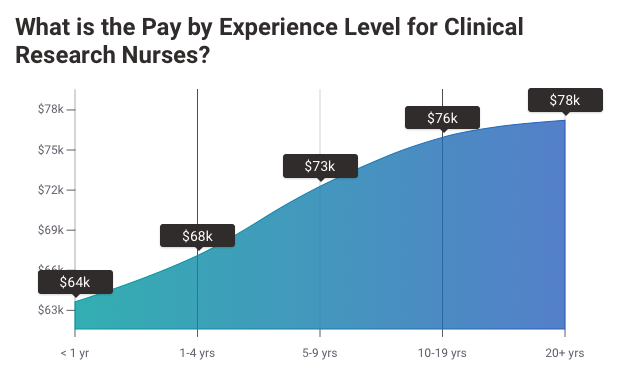
CNR: What Should Clinical Research Nursing Students be Reading
As an aspiring clinical research professional, it is important to stay up to date with what is happening in your field. Clinical Nursing Research (CNR) is a journal that covers clinical nursing. It is released four times a year and addresses clinical issues meaningful to nursing students. It also opens an international forum provide room to:
Spread widely findings of a particular research to practicing nurses.
Improve clinical practice by identifying clinical application that conforms to the latest research.
Encourage clinical practitioners among themselves.
CNR is one of the most important resources for clinicians and researchers who are concerned with improving their work. In addition, CNR provides graduates and undergraduates in the field of clinical nursing research with a source of field insights that will impress future employers and coworkers.
Features involved
Clinical nursing research offers some articles and features such as:
Feature articles with international references: this will enhance your understanding of the full scope of international research.
Articles researched: This involves discussions on a research project’s findings
Brief your research: short research reports on specific aspects of practice.
Replicated studies: These studies compare new findings with the existing ones, thereby providing important information to clinicians. This forms the basis for introduction of findings.
Who does the research?
CNR is written by a clinical nurse specialist. Their aim is to increase the standard of patient care. They also focus on finding a new treatment for different diseases. The nurses make sure that the patients are well protected and well supported during the period of study.
At CCRPS, in addition to ACCRE-certified courses, we also offer in-depth guide on how you can succeed in the clinical research field. Check below to sample some of our nursing articles.
Take courses from CCRPS and learn more on how to become a clinical research professional.
At CCRPS, we offer ACCRE-certified courses to help you excel in the clinical research field. Enhance your skills and knowledge with our comprehensive courses:
Clinical Cancer Research and Areas That Hold Interest
Clinical Cancer Research is a peer-reviewed medical journal based on oncology. It includes therapy, diagnosis, prevention, molecular and cellular characterization of human cancer. It also covers topics like clinical generics, surgical oncology, pathology, pediatric oncology, radiation therapy, and hematological oncology.
What it entails
The publication elaborates on the application of different disciplines of molecular, cell biology, and immunology genetics in the integration of cancer in humans. The main areas of interest are the clinical trials, which evaluate of the new treatments available to predict resistance and response.
Animal and laboratory studies of molecule targeted agents and drugs that have potential too. It focuses on studies of metastatic disease, malignant phenotype progression, and oncogenesis of targetable mechanisms.
The journal issues and what they cover
Clinical Cancer Research has been in publication since 1995. Publications have been submitted by all sorts of investigators, who represent disciplines like clinical and laboratory all over the world. Countless translational and innovative clinical cancer studies have been published in order to bridge clinic and laboratory. This is a journal that holds interest in the clinical trials that evaluate any new treatment. This is especially true when it comes to pharmacology research and molecular alterations.
Other interest areas include:
Human tumors molecular characterization
Radiation and radiobiology oncology
Gene therapy
Clinical and immunotherapy immunology
Application of biostatistics and bioinformatics personalized medicine
Pharmacogenomics and pharmacogenetics
Expand your knowledge and expertise in oncology research with CCRPS courses:
Clinical Research Coordinator: Course Link
Pharmacovigilance Certification: Course Link
CRA (Clinical Research Associate): Course Link
ICH-GCP (International Conference on Harmonization - Good Clinical Practice): Course Link
Clinical Trials Assistant Training: Course Link
Advanced Clinical Research Project Manager Certification: Course Link
Advanced Principal Investigator Physician Certification: Course Link
Medical Monitor Certification: Course Link
Take courses from CCRPS and learn more on how to become a clinical research professional.
Discover more from Clinical Research Training | Certified Clinical Research Professionals Course
Understanding the Scope of Clinical Research and What it Entails
Clinical research can be defined as a study of illness and health in humans. This is how we find out ways in which we can prevent, make a diagnosis, and even treat illnesses. Usually, the focus is on the improvement of knowledge and coming up with the best diagnostic methods or new treatments to create better care.
Clinical research respects a study protocol that is very precise and organized. A clinical research can only be fully realized under specific conditions.
The research must:
Collect the necessary consent from all participants of the research
Obtain all the approvals as required by the law and ensure all the ethical steps are adhered to
Take the necessary measures to protect everyone who joins in the research
Be conducted by people who are competent
Have the aim of growing medical knowledge
Types of clinical research
There are two types of clinical research that can be conducted:
1. Observational studies (epidemiology, cohort study)
2. Clinical trials or Intervention studies
1. Observational studies
This kind of research is aimed at the improvement of knowledge of a particular disease as well as how it has evolved over a period of time. Usually, they are conducted within a set framework where patients have follow ups in reference centers. Historical studies are also included here.
2. Clinical trials or Intervention studies
These aim at providing enough scientific evidence of the safety and effectiveness of new drugs, new illness management, or new care device. This is a step which is necessary before any molecule can be made a drug as well as a new medical device before it is brought to the market.
There are very strict steps that are involved in this case and they include:
Administration to healthy humans
Administration in the patients
Therapeutic efficacy
Marketing authorization or approval
Evaluation and monitoring of the side effects
Clinical trials
Clinical trials fall under the clinical research umbrella. It is an experiment which is designed to address very specific questions regarding possible treatments or any new ways that can be used with existing treatments. They are done to determine if the treatments and new drugs are effective and safe. Clinical trials are usually a long and careful process that can take years before completion. The treatment has to be studied in a lab by qualified doctors.
Drugs and treatments need to be animal tested before any human testing.
Medical care and clinical research
It is common for people to confuse medical care and clinical research. In Medicare, a patient has a plan that is unique to their needs. The treatment plans have already been tested and proven safe and effective.
Everyone who wants to undertake clinical trials should be aware that it is an experiment. This means that while patients will have access to the newest technology, clinical trial may or may not benefit you.
In clinical research, there is usually a pre-determined set plan or protocol that the researcher and the subject have to follow. In a clinical trial, there are steps taken if someone starts feeling unwell during the trials, but the researcher is not allowed to make any adjustments to the plan to fit any particular subject. It is therefore important to have everything laid out with the researcher and your doctor.
Take courses from CCRPS and learn more on how to become a clinical research professional.
Exploring the World of Clinical Research: Understanding Its Significance and Processes
Courses:
Clinical Research Coordinator: Gain insights into the role of Clinical Research Coordinators in organizing and overseeing clinical research studies.
Pharmacovigilance Certification: Understand the importance of pharmacovigilance in ensuring drug safety throughout clinical research processes.
CRA (Clinical Research Associate): Delve into the responsibilities and duties of Clinical Research Associates in managing and monitoring clinical trials.
ICH-GCP: Learn about the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH) Good Clinical Practice (GCP) guidelines essential for conducting ethical clinical research.
Clinical Trials Assistant Training: Acquire the foundational skills necessary to support clinical trial operations effectively as a Clinical Trials Assistant.
Advanced Clinical Research Project Manager Certification: Enhance your expertise in clinical research project management with advanced certification training.
Advanced Principal Investigator Physician Certification: Gain advanced certification tailored for Principal Investigator Physicians involved in clinical research studies.
Medical Monitor Certification: Explore the critical role of medical monitoring in ensuring the safety and integrity of clinical trials.
Discover more from Clinical Research Training | Certified Clinical Research Professionals Course
Answering Common Clinical Research Questions
Not many people know anything about clinical research; what it means, the purpose of clinical research etc. There are certain clinical research questions that are asked frequently. They need to be answered to give people assurance on what clinical research is really all about. Understanding these basic questions will help people make the right decisions concerning clinical research.
What does clinical research mean?
Clinical research are studies carried out to with the aim of improve human health and health care service in general. It seeks to answer difficult health questions, like how a sickness is contacted and spread, how to cure a sickness, and how to create new treatment methods.
Who can participate as research subjects?
Anyone can, as long as they are aware of the risks. A great way to find studies is visiting NIH clinical trials.
There are guidelines to choosing the people that can participate in a research as clinical research volunteers. These guidelines are called the “inclusion criteria” or the “eligibility criteria”. These criteria are based on factors such as gender, age, medical history, medical conditions, type of disease and stage of the disease etc. before a person can participate, they must have met the requirements to participate.
What are the benefits that a person can get from participating in a clinical research?
There are various benefits that a subject can get from volunteering as a participant. They are:
They get to play important roles and make important decisions that pertain to their health.
They have access to new treatments that are not yet available to the general public.
They have access to expert care during the trials for free.
They make valuable contributions to the area of medical science without necessarily being medical experts.
Some clinical researches offer monetary benefits, reduced treatment cost or even free medical care to the participants to serve as incentives.
How can I get comprehensive clinical research training?
This question is usually asked by those who have developed interest in the area of clinical research and might want to start a clinical research career. There are quite a lot of avenues through which you can get the required training. But the best place where you can take the right clinical research training courses is at ccrps.org. Receive education and knowledge from experts in the industry and succeed in your chosen career.
Take courses from CCRPS and learn more on how to become a clinical research professional:
Clinical Research Opportunities in New York City
Clinical research in New York has been on the rise in recent years. New York City is known for its advancement in clinical research and management.
Participating in a clinical research in New York is beneficial to the participants and it is a way for you as the participant to help people with such health issues and also contribute to the medical sciences. Popular benefits include:
Getting free medical care.
Being involved actively.
It helps you to improve your lifestyle for better health.
You have access to and benefit from new treatments that are not yet available for the general public.
Clinical research in US have been ongoing for a long time. With every discovery and advancement, it becomes more important part of healthcare. If you are interested in being a clinical researcher in US, you can get all the help that you will need to be successful at CCRPS. From knowledge to experience to degree and certification, you will get all the needed help from experts and professionals in the clinical research industry that are willing and ready to set you on the right path.
Interested in pursuing a career in clinical research? Explore the array of courses offered by CCRPS:
Clinical Research Coordinator: Learn more about coordinating clinical research studies. Clinical Research Coordinator Course
Pharmacovigilance Certification: Dive into the essentials of pharmacovigilance. Pharmacovigilance Certification Course
CRA (Clinical Research Associate): Explore the responsibilities and skills required for a CRA role. CRA Course
ICH-GCP (International Conference on Harmonisation of Good Clinical Practice): Understand the guidelines for conducting clinical trials. ICH-GCP Course
Clinical Trials Assistant Training: Gain expertise in assisting with clinical trials. Clinical Trials Assistant Training Course
Advanced Clinical Research Project Manager Certification: Elevate your career with advanced project management skills. Advanced Clinical Research Project Manager Certification Course
Advanced Principal Investigator Physician Certification: Take your expertise as a physician to the next level in clinical research. Advanced Principal Investigator Physician Certification Course
Medical Monitor Certification: Enhance your skills as a medical monitor in clinical trials. Medical Monitor Certification Course
Empower yourself with the knowledge and skills needed to excel in the field of clinical research through CCRPS. Explore our Clinical Research Training programs today!
Clinical Research Field Knowledge Everyone Should Know
What is Clinical Research?
Clinical research is a branch under healthcare sciences that is responsible for finding out the effectiveness and safety of medical devices, medications, diagnostic products and treatment regimens that are intended for human use in the cure of diseases and illnesses.
Clinical research is used for the diagnosis, prevention, treatment, relieving or alleviating of symptoms of a disease, and is much different from clinical practice. The major difference between clinical practice and clinical research is this - clinical practice works with only established treatments, while clinical research works to make a new established treatment.
Clinical research spans from inception tests to introduction to the consumer market. It goes through stages of tests called phases. It is done with the main purpose of testing the effectiveness and safety of a drug for human use.
Bodies Concerned With Clinical Research
Europe
In the European countries, the European Medicines Agency (EMA) is the regulatory body that facilitates clinical research studies. Human subjects that have given their informed consent are allowed to participate as research subjects in the 4 phases of clinical research.
United States
In the United States, the conduction of clinical research is a little more complex.
A new drug or treatment that has not yet been approved or cleared by the Food and Drug Administration (FDA) cannot be given a license. Clinical research data has to be submitted to the FDA through the support of an (IND) Investigational New Drug application. This enables the FDA to review the new treatment before they are used to conduct studies involving human subjects. After all proper investigations by the FDA, the new drug or treatment can now be licensed and advertised.
In the case of medical devices, if the device shows signs of significant risk or has not been exempted or licensed by the FDA, then the clinical research data has to be submitted to the FDA through the support of an (IDE) Investigational Device Exemption application. This process may also require approval from any of the following; Institutional committee reviews, Radiation Safety Committee, Conflict of Interest Committee, Privacy Board, Radioactive Drug Research Committee, Research Ethics Board (REB) or the Institutional Review Board (IRB).
Criteria for Review
The criteria for the review of all clinical research depends on the following;
• Federal regulations that the research falls under and is subjected to.
• The regulations the institutions subscribe to.
• The response to local, state, federal laws, policies, rules, regulations, or accreditation entity recommendations.
The last criteria for review is supervised by the IRB or ERB in concordance with the regulatory affairs to protect human subjects.
Where Do Clinical Researches Take Place?
Clinical researches usually take place at affiliated research study sites, hospitals, healthcare clinics, or academic medical centers. These places are strategically placed to provide the competence of the academic institutions involved as well as provide closeness to the medical participants pool.
The ethical issues and conducts of clinical research is overseeing and supervised by their internal institutional review boards.
There are lots of professionals involved in the world of clinical research, as it is a complex network of pharmaceutical companies, academic research institutions, and biotechnology companies. All these has led to the fast growth of technologies used in the operational factors and management aspects of clinical research.
The operational factors are overseen by clinical research professionals such as, CRAs, CTAs, CRCs, and others, while the management aspects of clinical research are overseen by CTMs, CRMs, CRPMs, CrDMs, and others.
Interested in pursuing a career in clinical research? Explore the array of courses offered by CCRPS:
Clinical Research Coordinator: Learn more about coordinating clinical research studies. Clinical Research Coordinator Course
Pharmacovigilance Certification: Dive into the essentials of pharmacovigilance. Pharmacovigilance Certification Course
CRA (Clinical Research Associate): Explore the responsibilities and skills required for a CRA role. CRA Course
ICH-GCP (International Conference on Harmonisation of Good Clinical Practice): Understand the guidelines for conducting clinical trials. ICH-GCP Course
Clinical Trials Assistant Training: Gain expertise in assisting with clinical trials. Clinical Trials Assistant Training Course
Advanced Clinical Research Project Manager Certification: Elevate your career with advanced project management skills. Advanced Clinical Research Project Manager Certification Course
Advanced Principal Investigator Physician Certification: Take your expertise as a physician to the next level in clinical research. Advanced Principal Investigator Physician Certification Course
Medical Monitor Certification: Enhance your skills as a medical monitor in clinical trials. Medical Monitor Certification Course
Empower yourself with the knowledge and skills needed to excel in the field of clinical research through CCRPS. Explore our Clinical Research Training programs today!
Discover more from Clinical Research Training | Certified Clinical Research Professionals Course
How To Maximize Using The Clinical Research Wiki
Exploring clinical research questions often leads individuals to valuable resources like the clinical research wiki, where comprehensive information regarding various aspects of clinical research is readily available. From understanding the definition of clinical research to delving into its different fields and professional roles, the wiki serves as a rich source of knowledge.
For instance, if you're curious about defining clinical research, the wiki elucidates it as a vital branch of healthcare science dedicated to assessing the safety and efficacy of medications, devices, diagnostic products, and treatment regimens intended for human use.
While the wiki serves as a useful starting point, it's essential to exercise caution as its content can be subject to change. Verifying information through the source material cited at the bottom of the page ensures accuracy and reliability.
Mastering the utilization of resources like the clinical research wiki is invaluable, particularly for individuals embarking on a career in clinical research. Whether aspiring to become a clinical research assistant, associate, or coordinator, meeting basic requirements such as obtaining a degree or certification is essential.
For those seeking assistance in navigating certification examinations conducted by the Association of Clinical Research Professionals (ACRP), CCRPS offers tailored support and quality clinical research programs. By enrolling in CCRPS courses, individuals gain the necessary knowledge and skills to embark on a successful career in clinical research.
Explore CCRPS courses to kickstart your journey in clinical research:
Clinical Research Coordinator: Gain insights into coordinating clinical research studies. Clinical Research Coordinator Course
Pharmacovigilance Certification: Deepen your understanding of pharmacovigilance essentials. Pharmacovigilance Certification Course
CRA (Clinical Research Associate): Learn about the responsibilities and skills required for a CRA role. CRA Course
ICH-GCP (International Conference on Harmonisation of Good Clinical Practice): Explore guidelines for conducting clinical trials. ICH-GCP Course
Clinical Trials Assistant Training: Enhance your skills in assisting with clinical trials. Clinical Trials Assistant Training Course
Advanced Clinical Research Project Manager Certification: Elevate your career with advanced project management skills. Advanced Clinical Research Project Manager Certification Course
Advanced Principal Investigator Physician Certification: Enhance your expertise as a physician in clinical research. Advanced Principal Investigator Physician Certification Course
Medical Monitor Certification: Develop proficiency as a medical monitor in clinical trials. Medical Monitor Certification Course
Empower yourself with the necessary tools and knowledge to excel in the dynamic field of clinical research through CCRPS courses. Start your journey today!
How to Get into Clinical Research Step by Step
Looking for clinical research jobs? Visit CCRPS at here you can get the all information about clinical research remote jobs. Apply now!
What is Clinical Research?
Clinical research is a branch under the healthcare sciences that is responsible for finding out the effectiveness and safety of medical devices, medications, diagnostic products and treatment regimens that are intended for human use to help cure illnesses. Clinical research is important for the diagnosis, prevention, treatment, alleviating of symptoms of a disease, but it is much different from clinical practice.
You want to get a career for yourself in clinical research, then let's take you through the drill.
The first step is to get an education.
Earn a bachelor's degree in a life science or health related discipline. Courses like medicine, pharmacology, biology, molecular biology, genetics, anatomy, biotechnology, nursing, physiology, chemistry, or bioengineering will equip you with the necessary and relevant knowledge to get you into clinical research.
Supplement your education by applying for the courses offered at your university or from professional organizations like Certified Clinical Research Professionals (CCRPS) and Association of Clinical Research Professionals (ACRP). These courses will include; study designs, Good Clinical Practices (GCP), research ethics, drug development cycle, regulatory affairs and requirements both in the US and internationally, among others.
Get a certification from a reputable organization, such as CCRPS, ACRPS, and Society of Clinical Research Associates (SOCRA). Make sure you study the ICH GCP guidelines and ethics to make you competent. Training in the International Conference on Harmonisation (ICH) Good Clinical Practices (GCP) ethics and guidelines improves your chances greatly. At CCRPS, we offer a free ICH GCP certification to help you get started.
Keep proper documented records of your certifications as well as your education.This will save you a lot of time and stress.
The next step is to gather experience.
The following are ways you can gather experience:
Volunteer - Look for volunteering opportunities around your area and volunteer to help with the projects that will be carried out in the clinical research industry. This helps you get closer to the professionals as well as the tasks itself. You can volunteer at clinical research professionals organizations related to the clinical research field or medical field, medical centers or hospitals, International Review Boards (IRB) or Research Ethics Committees.
Research Projects - Take up clinical research monitoring projects and you can gain at least two years of experience.
Internships - Seek out an internship with medical firms, biotechnology companies, and pharmaceutical companies while you're still in college. CCRPS offers an internship program that can help you develop your resume.
The next step after getting an education and gathering experience is to apply for entry-level positions
A job as a clinical research professional is a rewarding career, and as such you must not only get an education and experience, but you must also be able to put in a high quality CV and cover letter for your application.
As long as you have a bachelor's degree and at least one year of experience in clinical research, you can easily qualify for an entry level position in clinical research. A quick search for “clinical research jobs near me” allows you gain more insight into the clinical research industry.
So, how do you get a job?
Here are tips that you need to keep in mind when you are applying for clinical research jobs.
• Be realistic about the jobs you can go into with your educational background and experience. Apply for entry-level positions firsts, then work your way to the top with targeted efforts and tenacity.
• Read each job descriptions clearly and highlight on your CV the relevant experience that you have that matches the specific role requirements. That little edit can change the way clinical research recruiters and consultants look at your CV as compared to others.
• Network with hiring managers and clinical research recruiters to expand your base as not all companies will advertise their vacancies. Upload your CV to a database that enables employers, clinical research recruiters, and hiring managers to easily find you.
Take courses from CCRPS and learn more on how to become a clinical research professional.
Clinical Research Coordinator: Gain foundational knowledge and skills essential for coordinating clinical research studies.
Pharmacovigilance Certification: Understand pharmacovigilance practices crucial for ensuring drug safety in clinical research settings.
CRA (Clinical Research Associate): Dive into the responsibilities and duties of Clinical Research Associates in managing and monitoring clinical trials.
ICH-GCP: Learn about the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH) Good Clinical Practice (GCP) guidelines essential for ethical clinical research.
Clinical Trials Assistant Training: Acquire the necessary skills to support clinical trial operations effectively.
Advanced Clinical Research Project Manager Certification: Elevate your expertise in clinical research project management.
Advanced Principal Investigator Physician Certification: Gain advanced certification tailored for Principal Investigator Physicians involved in clinical research studies.
Medical Monitor Certification: Explore the critical role of medical monitoring in ensuring the safety and efficacy of clinical trials.
Discover more from Clinical Research Training | Certified Clinical Research Professionals Course
Clinical Research vs Clinical Trial
Understanding the nuances between clinical research and clinical trials is paramount for aspiring clinical research professionals. While these terms are often used interchangeably, it's essential to grasp their distinctions to navigate the field effectively.
Exploring Clinical Research and Clinical Trials
Clinical Research Coordinator Training
Embark on your journey into the realm of clinical research with our specialized Clinical Research Coordinator course. Gain insights into the fundamental principles of research coordination, setting a solid foundation for your career aspirations.
Pharmacovigilance Certification
Ensure safety and efficacy remain at the forefront of clinical trials with our comprehensive Pharmacovigilance Certification course. Learn to uphold stringent pharmacovigilance standards, safeguarding patient welfare throughout the research process.
CRA Training
Equip yourself with the essential skills needed to thrive in clinical research roles through our dynamic CRA course. Delve into regulatory compliance and monitoring techniques, preparing you to excel as a Clinical Research Associate.
Bridging the Gap: Clinical Research vs. Clinical Trials
While clinical research encompasses a broad spectrum of scientific inquiry into human health, clinical trials represent a specific subset within this realm. Clinical trials serve as structured experiments aimed at evaluating the safety and efficacy of new treatments or treatment modalities.
ICH-GCP Compliance
Adherence to Good Clinical Practice (GCP) guidelines is paramount for the integrity and credibility of clinical trials. Explore the principles of ICH-GCP through our specialized ICH-GCP course, ensuring your research endeavors adhere to the highest ethical and regulatory standards.
Clinical Trials Assistant Training
Facilitate the seamless execution of clinical trials with our comprehensive Clinical Trials Assistant Training. Acquire the practical skills and knowledge needed to support research teams effectively throughout the trial lifecycle.
Empowering Your Clinical Research Career
As you embark on your journey toward a rewarding career in clinical research, leverage the expertise and resources available at ccrps.org. Our courses are meticulously designed to equip aspiring professionals with the requisite skills and knowledge to thrive in this dynamic field.
For those looking to further advance their expertise, consider our Advanced Clinical Research Project Manager Certification and Advanced Principal Investigator Physician Certification. These programs are designed to deepen your understanding and enhance your capabilities in leading complex research projects.
Take the first step towards realizing your clinical research ambitions by enrolling in our specialized courses at CCRPS. Explore the intricacies of clinical research, enhance your understanding of clinical trials, and unlock a world of opportunities in the realm of healthcare innovation.
How to Get into Clinical Research
If you want to get into the clinical research, there are a few steps you will have to go through. In this article, we'll be taking you through the steps and some common questions.
What does Clinical Research entail?
Clinical research entails testing medicines or products for safety and effectiveness. It involves working with patients during extended experiments to record and quantify the effect that different medicines produce. It is a highly regulated field due to the use of human subjects.
Salary earned ranges from $39,000 to $87,000. However, the more one acquires certifications and qualifications, the more opportunities you have. That means you can choose the best position and salary for you.
The first step is to get an education.
Earning a bachelor's degree in a life science or health related discipline by taking specific courses like medicine, pharmacology, biology, molecular biology, genetics, anatomy, biotechnology, nursing, physiology, chemistry, or bioengineering will equip you with the necessary and relevant medical knowledge, science, and technicalities to qualify you into practicing as a clinical research.
Taking courses that are relevant to the clinical research that will give you the necessary experience and knowledge that are relevant to clinical research conducts. Applying for the courses offered at your university or from professional organizations like Certified Clinical Research Professionals (CCRPS) and Association of Clinical Research Professionals (ACRP) are a great place to start. These courses will include topics such as study designs, Good Clinical Practices (GCP), research ethics, drug development cycle, regulatory affairs and U.S as well as international requirements.
You can get a certification from a reputable organization, such as CCRPS, ACRPS, and Society of Clinical Research Associates (SOCRA), as long as you have a Bachelor's design and at least one year of experience in clinical research. This certification allows you gain more access into the clinical research industry.
When you are taking your courses, make sure you study the ICH GCP guidelines and ethics thoroughly. Training in the International Conference on Harmonisation (ICH) Good Clinical Practices (GCP) ethics and guidelines improves your chances of getting hired greatly.
Remember to keep proper documented records of your certifications as well as your education. This will save you a lot of time and stress.
The next step is to gather experience.
The following are ways you can gather experience;
Volunteer - Look for volunteering opportunities around your area and volunteer to help with the projects that will be carried out in the clinical research industry. This helps you get closer to the professionals as well as what to expect at the job. You might be chanced to start out as a clerical worker or a data entry staff, but not to worry, you'll be able to work your way up the ladder. Once you are in the field, you can discuss the possibility of applying for a position with the place you are working at in the future. Employers will be more likely to consider you when there is someone in the company vouching for you.
You can volunteer at clinical research professionals organizations related to the clinical research field or medical field, medical centers or hospitals, International Review Boards (IRB) or Research Ethics Committees.
Research Projects - Most entry level jobs require around two years of experience. Taking up clinical research monitoring projects for a few years can really help you get the experience you need. You can also conduct research studies with human subjects during your pursuit of a bachelor's degree or graduate degree.
Internships - Seek out an internship with medical firms, biotechnology, and pharmaceutical companies while you're still in college. You may or may not get paid as an intern but it's nothing compared to the experience you'll gain that will be needed for your venture into clinical research.
Finally, the last step is to apply for entry-level positions.
This is the last step that will get you right into the world of clinical research. After all your education and gathered experience, you cam apply for an entry-level position as a Clinical Research Coordinator (CRC) or a Clinical Trial Assistant (CTA). Both positions only require around two years of experience and they are the ones that you can qualify for. Applying for high-level positions you don’t qualify for yet will only waste your time.
Generally, you should apply for positions at smaller firms. It's okay to aim for positions at the biggest pharmaceutical companies and clinical research organisations (CROs), but as a newcomer, the competition may just be too high. So, why not just apply for positions at smaller firms and work your way to the top?
Take courses from CCRPS and learn more on how to become a clinical research professional.
Discover more from Clinical Research Training | Certified Clinical Research Professionals Course
Joining the ICH-GCP Clinical Research Program
Clinical research is one of the best career path to get involved in. They have positions for every lifestyle and personality. However, a solid understanding of the rules and regulation of the field is critical for every professional in the field. In this article, we will talk about the GCP and how it should impact the way you approach clinical research.
About the GCP
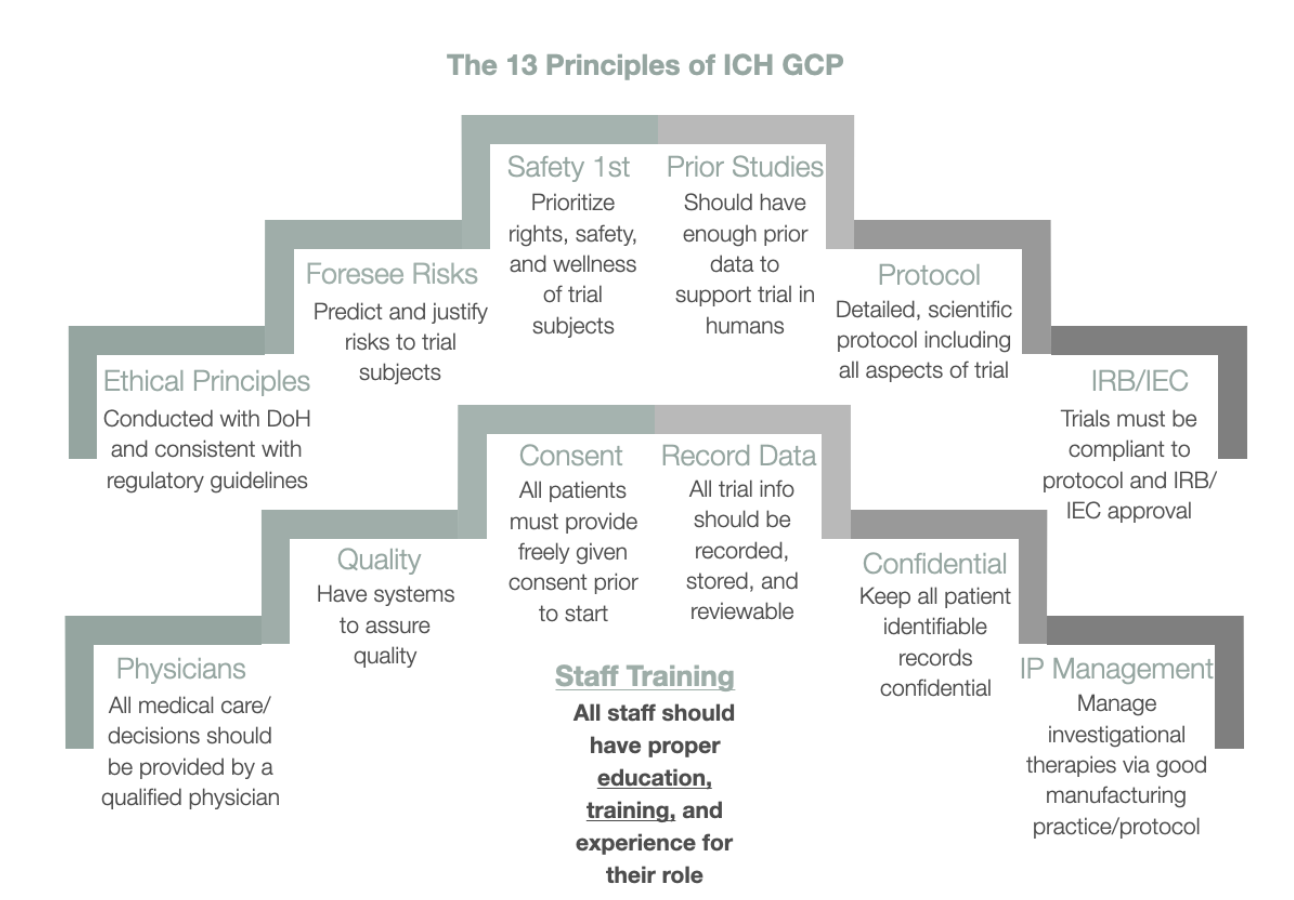
Good Clinical Practice (GCP) is an international standard provided by the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH). GCP enforces strict guidelines on ethical aspects of the clinical study.
Individuals who are aware of the significance of very good clinical practice guidelines and the role of the clinical research know the importance of online GCP programs.
Medical professionals who have successfully completed their clinical research training program can improve their expertise on regulatory authorities and approval specifications for the marketing of the healthcare products. This can make a big change in their work life. A good training program can help them improve their proficiency in the best use of medical data, databases and data evaluation. This will help them build proficiency in the communication, research and management, educational approaches, problem-solving and other things related to the clinical research.
Make a better informed decision
The salary is one of the main factors considered by people planing to choose a degree or certification program. Here, you can find the average clinical research salary in your area and decide on whether a career in the clinical research.
If you wish to become a certified clinical research professional, then you have to find out and join in the program specially designed for career.
Clinical research involves drug development as well as healthcare research. Experts in this profession engage in the patient-focused research and make use of every chance to be successful in the clinical research career.
CCRPS visitors learn about clinical research and are able to make an informed decision to join the best program for them. When you have questions, you can have instant assistance from the experienced and committed professionals. We ensure our students understand about everything associated with the clinical research degree program and use every opportunity to be successful in the clinical research sector.
Improve your resume now with online clinical research certification or membership in clinical research societies.
Relevant Course Links
For individuals eager to excel in clinical research, enrolling in specialized courses can provide a significant advantage. Here are some relevant courses offered by CCRPS:
Empowering Your Clinical Research Journey
At CCRPS, we empower aspiring clinical researchers to make informed decisions about their education and career paths. Our programs provide comprehensive insights into the clinical research sector, ensuring that students are well-prepared to thrive in this dynamic field. With experienced professionals dedicated to your success, you'll have the support you need to excel in clinical research.
Take courses from CCRPS and learn more on how to become a clinical research professional.
Discover more from Clinical Research Training | Certified Clinical Research Professionals Course
What Is a Clinical Research Coordinator
The clinical research is a ground breaking field in the world of medical advancement and as such, has a wide variety of professionals that are changing and channeling the drive.
We will be taking a look at one of the highest paying certified clinical research professionals, clinical research coordinator.
CLINICAL RESEARCH COORDINATOR
The clinical research coordinator is responsible for conducting trials as per the GCP guidelines under the supervision of the Principal Investigator (PI). Although the PI carries the overall responsibility for performing the trial, the CRC is the heart and soul of the clinical trial and that, ultimately, it is the CRC who carries ahead the research objectives and ensures the success of the clinical trial.
Having a certification in clinical research in other to become a clinical research coordinator is not compulsory, but certification enables one to show that they have met the necessary requirements and have gained job-relevant knowledge and skills. This distinction is very important to pharmaceutical companies and contract research organizations, who frequently hire clinical research staff. Consider enhancing your qualifications with a Clinical Research Coordinator certification from CCRPS.
EDUCATIONAL REQUIREMENTS
• High school diploma and 6,000 hours of experience.
• An associate degree in clinical research fields amd 4,500 hours of experience, or
• A Bachelor's, Master's, or Registered Nurse degree and 3,000 hours of clinical research experience.
You can come from a variety of medical sciences or health related fields, or from a nursing background as a RN. Courses offered in hospital and clinical related ethics, team management, and research methodologies will be especially valuable. Explore further with courses like ICH-GCP, Clinical Trials Assistant Training, and Advanced Clinical Research Project Manager Certification to deepen your expertise.
Who Will Hire Clinical Research Coordinators?
Pharmaceutical Organizations
Contract Research Organizations
Universities
Hospitals
SPECIALIZATIONS
They focus on the following;
1) Participating in preparation and management of research budgets and monetary disbursements.
2) Informing patients or caregivers about study aspects and outcomes to be expected.
3) Communicating with laboratories or investigators regarding laboratory findings.
4) Ordering drugs or devices necessary for study completion.
5) Directing the requisition, collection, labeling, storage, or shipment of specimens.
6) Arranging for research study sites and determine staff or equipment availability.
7) Reviewing scientific literature, participate in continuing education activities, or attend conferences and seminars to maintain current knowledge of clinical studies affairs and issues. You can also gain specific certifications like Pharmacovigilance Certification and Medical Monitor Certification to further specialize in your field.
SALARY
Clinical research coordinators have had a positive trend of pay for experience. Therefore a CRC with less than 5 years experience is paid an average total compensation of $43,000 (based on 1,237 salaries). Employees with 5 to 10 years experience can expect the average compensation of $51,000 (based on 429 salaries). Employees with 10 to 20 years of experience is paid an average compensation of $55,000 (based on 265 salaries). Finally, employees with more than 20 years of experience can expect a compensation of $62,000 (based on 52 salaries).
While many stay CRCs their entire career, most will move to higher postions after 3-5 years of experience. One of the most popular careers CRCs switch to is CRA, which can pay up to $300k. Prepare for this role with a CRA certification from CCRPS.
Take courses from CCRPS and learn more on how to become a clinical research professional.
Discover more from Clinical Research Training | Certified Clinical Research Professionals Course