What Is a Clinical Research Coordinator
The clinical research is a ground breaking field in the world of medical advancement and as such, has a wide variety of professionals that are changing and channeling the drive.
We will be taking a look at one of the highest paying certified clinical research professionals, clinical research coordinator.
CLINICAL RESEARCH COORDINATOR
The clinical research coordinator is responsible for conducting trials as per the GCP guidelines under the supervision of the Principal Investigator (PI). Although the PI carries the overall responsibility for performing the trial, the CRC is the heart and soul of the clinical trial and that, ultimately, it is the CRC who carries ahead the research objectives and ensures the success of the clinical trial.
Having a certification in clinical research in other to become a clinical research coordinator is not compulsory, but certification enables one to show that they have met the necessary requirements and have gained job-relevant knowledge and skills. This distinction is very important to pharmaceutical companies and contract research organizations, who frequently hire clinical research staff. Consider enhancing your qualifications with a Clinical Research Coordinator certification from CCRPS.
EDUCATIONAL REQUIREMENTS
• High school diploma and 6,000 hours of experience.
• An associate degree in clinical research fields amd 4,500 hours of experience, or
• A Bachelor's, Master's, or Registered Nurse degree and 3,000 hours of clinical research experience.
You can come from a variety of medical sciences or health related fields, or from a nursing background as a RN. Courses offered in hospital and clinical related ethics, team management, and research methodologies will be especially valuable. Explore further with courses like ICH-GCP, Clinical Trials Assistant Training, and Advanced Clinical Research Project Manager Certification to deepen your expertise.
Who Will Hire Clinical Research Coordinators?
Pharmaceutical Organizations
Contract Research Organizations
Universities
Hospitals
SPECIALIZATIONS
They focus on the following;
1) Participating in preparation and management of research budgets and monetary disbursements.
2) Informing patients or caregivers about study aspects and outcomes to be expected.
3) Communicating with laboratories or investigators regarding laboratory findings.
4) Ordering drugs or devices necessary for study completion.
5) Directing the requisition, collection, labeling, storage, or shipment of specimens.
6) Arranging for research study sites and determine staff or equipment availability.
7) Reviewing scientific literature, participate in continuing education activities, or attend conferences and seminars to maintain current knowledge of clinical studies affairs and issues. You can also gain specific certifications like Pharmacovigilance Certification and Medical Monitor Certification to further specialize in your field.
SALARY
Clinical research coordinators have had a positive trend of pay for experience. Therefore a CRC with less than 5 years experience is paid an average total compensation of $43,000 (based on 1,237 salaries). Employees with 5 to 10 years experience can expect the average compensation of $51,000 (based on 429 salaries). Employees with 10 to 20 years of experience is paid an average compensation of $55,000 (based on 265 salaries). Finally, employees with more than 20 years of experience can expect a compensation of $62,000 (based on 52 salaries).
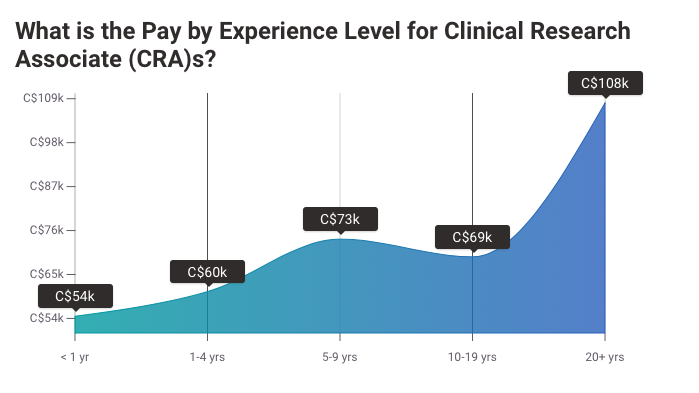
While many stay CRCs their entire career, most will move to higher postions after 3-5 years of experience. One of the most popular careers CRCs switch to is CRA, which can pay up to $300k. Prepare for this role with a CRA certification from CCRPS.
Take courses from CCRPS and learn more on how to become a clinical research professional.
Discover more from Clinical Research Training | Certified Clinical Research Professionals Course
Job Training for High School Graduates Using a Clinical Research Institute for Online Clinical Research Certification (CTA, CRA, CRC)
Choosing the right job course online is a challenging task for almost everyone new to certification programs. There are so many different attention-grabbing packages of affordable clinical research programs, how do you know which one is for you? People who focus on the successful institution specializing in the clinical research programs are most likely to find a program they can truly learn and benefit from. Visit CCRPS, a major AACRE accredited U.S Organization, and find courses provided by the certified clinical research professionals. You will get guidance about the programs in the clinical research that goes beyond your expectations. Their education will help you make a better-informed decision to choose and join in one of the best programs without complexity in any aspect.
Job training for high school graduates
Future professionals with an ambition to choose and join the best courses should consider important things like the overall value of the course and how they contribute to career development. At CCRPS, we not only offer a description of what to expect in the field, we also offer real life suggestions from experts already working in clinical research.
Once you have joined our program, you can learn more than a few important things and enhance your career profile. You will get different career options and make positive changes in your career life. You will be satisfied with the successful method to prefer and join in the suitable job after a comprehensive analysis of an array of important things.
Here are some courses you might consider:
Clinical Research Coordinator Certification - Ideal for those looking to coordinate clinical trial activities.
Pharmacovigilance Certification - Focuses on drug safety and adverse effects management.
CRA Certification - Prepares you for the role of Clinical Research Associate.
ICH-GCP Certification - Essential for professionals needing to understand Good Clinical Practice guidelines.
Clinical Trials Assistant Training - Provides foundational knowledge for those starting in clinical trials.
Advanced Clinical Research Project Manager Certification - For experienced professionals aiming for project management roles.
Advanced Principal Investigator Physician Certification - Tailored for physicians leading clinical trials.
Medical Monitor Certification - Designed for those overseeing the medical aspects of clinical trials.
Take courses from CCRPS and learn more on how to become a clinical research professional.
Discover more from Clinical Research Training | Certified Clinical Research Professionals Course
Why Do People Choose Online Clinical Training Courses?
People wish to have the perfect platform to perfect their clinical research. As the clinical training program courses become more popular among people, in some cases there are people who simply choose clinical research training courses without knowing its roles and responsibilities.
When people decide to pursue clinical research course, there are several available ways, such as the Clinical Research Coordinator and Pharmacovigilance Certification courses offered at CCRPS.
Although there are many clinical research institutes, most prefer taking clinical research training programs to make their career stronger in the medical field. This is mainly because when people choose courses in clinical research institution or any research centers, it would costs $30,000 to $ 50,000 while online courses like the CRA and ICH-GCP certifications cost much less.
Facilities provided in clinical research training in institutes:
You must be wondering how an online course might differ from a physical course. Online training courses are very similar to the courses offered in clinical research institutes. People can choose their desired specialization and attend daily sessions. Once they have completed all sessions and passed the course examination, they receive a course completion certificate such as the Clinical Trials Assistant Training.
Although the online clinical courses are similar to the courses offered in clinical research institutes, there are some facilities which are offered only in the clinical research institutes and research centers:
In clinical training institute, students can experience clinical research operations with patients.
Students are allowed to do test on various drugs and medicine, analysis them in traits test center.
Based on the analysis test report, student can learn to decide whether the drugs are safe for market use or need to be sent for further analysis.
These facilities are only provided to people that choose clinical research courses from clinical research centers and institutes. These skills and experiences might be important to potential employers, so be sure to check the requirements on all job postings. For more details regarding this you can check on Advanced Clinical Research Project Manager Certification and Advanced Principal Investigator Physician Certification or visit us to learn more about our Medical Monitor Certification.
Take courses from CCRPS and learn more on how to become a clinical research professional.
Discover more from Clinical Research Training | Certified Clinical Research Professionals Course
What Made Clinical Research Courses Popular in the Medical Field?
When students wish to have a strong career in the interesting medical field, they often become clinical researchers. The clinical researcher career is a popular choice because it brings several career as well as financial benefits. However, when you decide to choose clinical researcher as a career, then you should be aware of the roles and responsibilities involved in them.
Since there are so many available clinical research training programs, it is important that people know what roles and what specialization they want to go into. For example, when people complete their course or training programs from licensed centers, they could get clinical research organization jobs in pharmaceutical companies, medical research organizations, and in clinics. Although there are many courses available, people who knew going into training knowing exactly where they wanted to work would benefit more from their classes. You can start with a specific role by enrolling in the Clinical Research Coordinator course or explore specializations such as Pharmacovigilance Certification and CRA Training.
Due to the popularity of the field, those who get their Master’s in Clinical Research have an advantage. Their degree could get them better positions and pay than their counterparts. For those looking to solidify their knowledge of good clinical practices, the ICH-GCP course is essential.
Many people show an interest in completing their Master’s in Clinical Research, but in real life getting a Master’s in Clinical Research is not easy. In order to complete the course, people would need $30,000 to $50,000. Course from the top research centers would cost even more.
To tackle this situation, most people look to get their Master’s in Clinical Research online. Many wonder if online courses would be on par to trial centers training. The answer is that when students decide to do their Master’s in Clinical Research online, they find that the courses are very similar to classroom training.
Moreover, by taking online courses people can avoid several additional costs like travel, high tuition, and taking time off work. The only thing that is different is that in an online master’s course, people can’t experience hands-on training with patients, drugs, and medicines. This gap can be bridged by courses like Clinical Trials Assistant Training, which provide a comprehensive understanding of clinical trial processes.
When deciding to pursue an online masters, below are some other information you should consider:
There are many training centers that offer online Master’s in Clinical Research with low tuition fees. So people need to compare and choose the best among them.
Check whether the training center provides clinical research quality assurance training across all specializations. This speaks to the quality and comprehensiveness of the courses offered. Advanced courses like Advanced Clinical Research Project Manager Certification and Advanced Principal Investigator Physician Certification can be crucial for career advancement.
While comparing centers based on their courses, check whether the center would offer help with job placements too.
Before committing to anything, check jobs near you to see if an online course completion certificate is valid for the posting.
Thus based on all above factors, you can confidently choose the best clinical research training institute to pursue your Master’s in Clinical Research.
Take courses from CCRPS and learn more on how to become a clinical research professional.
Discover more from Clinical Research Training | Certified Clinical Research Professionals Course
Everything You Should Know About CTRI UCSD
The UC San Diego Altman Clinical and Translational Research Institute, better known as CTRI UCSD, acts as the hub for translational medicine. It helps in delivering the scientific breakthrough patient care through clinical trials.
They offer participants early access the treatments, which would one day re-define the standards of care. Every year, the clinical research volunteer program would enroll the participant with the hundreds of the clinical trials for promising the investigational drugs, devices and the treatments.
If you want to become part of a research institute like CTRI UCSD, clinical research education can help you.
Become well versed in the field that you really like.
Pave a way for you to shine and know about the field.
Helps you to do more research work based on the type of the field that you have chosen.
Things that you want to know about clinical research
The clinical research acts as the branch of the healthcare science. If you are interested in the field, doing the clinical research online training program that is associated up with clinical research associates (CRA), clinical research coordinators (CRC), or clinical trial assistants (CTA) could be a great way to get started.
Different research training programs could vary based on the student locations, time and the learning demands. However, CCRPS’ self paced online modules is reliable and contain interactive tutoring sessions that promotes monitored competency. Classes provide the foundation that will help you break into the field.
The ccrps.org paves a way for you to get trained up in the clinical research. Before choosing it, you can go through all the reviews that are available to give you a better idea of our courses. To know more details about it you can gather the information for training in clinical research here.
Take courses from CCRPS and learn more on how to become a clinical research professional. Explore our specialized certifications like Advanced Clinical Research Project Manager Certification, Advanced Principal Investigator Physician Certification, and Medical Monitor Certification.
Discover more from Clinical Research Training | Certified Clinical Research Professionals Course
Clinical Research Quality Assurance
For a career in clinical research quality assurance, understanding internal, national and international regulations in every aspect of clinical development is absolutely essential. Legal setbacks can be dangerous for patients, extremely costly, and highly damaging to a company’s reputation. Therefore, the role of the Good Clinical Practice QA professional is a high profile one and can offer excellent opportunities for development. However, it can be a tricky area to get into. Enhance your understanding and skills with an ICH-GCP course.
Just as in clinical operations, as your QA career develops you will normally become more office based; seeing to the work of audit teams, developing audit strategies and plans and ensuring a culture of compliance in your own organization as well as the ones you’re in partnership with.
You will also gain exposure to other areas of QA, such as Good Pharmacovigilance Practice (GPvP), Good Laboratory Practice (GLP) and Good Manufacturing Practice (GMP). Experience in more than one QA specialization will make you more attractive to potential employers. For instance, GPvP experience is particularly sought after these days. Consider enrolling in a Pharmacovigilance Certification to enhance your credentials in this area.
Many people choose to go down the interim path as their QA careers develop, opting for either short-term, ad-hoc work to complete individual projects for a range of clients or to take longer-term contracts that offer more security but at a lower pay rate.
The right personality is crucial to success in the QA world. Thankfully the days of QA groups being seen as ‘other’ in most companies are long gone.
You will be expected to train staff from a wide variety of disciplines, answer QA related queries and, on occasion, deal with some difficult situations. Very strong interpersonal and communications skills, a high degree of tact and knowledge in different areas is essential to the job. Expand your capability with Clinical Research Coordinator training or a Clinical Trials Assistant Training course.
HOW MUCH DOES A CLINICAL QUALITY ASSURANCE MANAGER MAKE?
The national average salary for a Clinical Quality Assurance Manager is $98,393. Salary estimates are based on 4,654 salaries submitted anonymously by clinical quality assurance manager employees.
Take courses from CCRPS and learn more on how to become a clinical research assurance manager. Specialized certifications like the Advanced Clinical Research Project Manager Certification or the Advanced Principal Investigator Physician Certification can propel your career forward.
Discover more from Clinical Research Training | Certified Clinical Research Professionals Course
Also, check out the CRA course for further qualifications, and the Medical Monitor Certification to explore other high-demand roles in clinical research.
Common Questions For a Clinical Research Project Manager
Get the right Clinical research project manager job with company ratings & salaries. Visit now!
Research project managers (PMs) are responsible for leading research projects to a defined business or scientific objective. To reach this goal, research project managers utilize different methodologies and techniques for managing and guiding the development of research instruments. They are also in charge of evaluating research related fieldwork, validating collected data, authoring reports and communicating across various research constituencies.
What responsibilities does a clinical project manager job have?
CPM jobs involve planning and managing all aspects of a clinical trial. The aim of clinical trials is to evaluate the safety and effectiveness of pharmaceuticals, medical devices, or in-vitro diagnostic devices to support regulatory submissions or marketing claims.
In order to manage a clinical trial adequately, CPMs manage a team of CRAs and Clinical Specialists. They also act as a link between the study sponsor and the clinical trial site. As a CPM, it is important to understand project management methodologies, like those taught in the Advanced Clinical Research Project Manager Certification. They will allow you to effectively lead a cross-functional team.
Analytical and writing skills are also very important, because a CPM job will involve developing documents such as protocols, informed consent documents, contracts, and grants. A skilled CPM should also be familiar with the financial aspects of a clinical study, including reviewing invoices, performing daily accounting tasks, and preparing budgets. Sometimes, clinical research manager jobs may include contributing to the statistical analysis and data reporting documentation used to support a marketing clearance, approval or registration.
How much do CPMs earn?
The average clinical research manager salary is $75,474 in the United States.
What is the demand for CPMs?
Demand for CPMs increases, as there are increased global regulations requiring more extensive clinical studies to support the safety and effectiveness claims of health-care products. CPMs may work for pharmaceutical, medical device, or in-vitro diagnostic device companies. CPMs may also work for contract research organizations (CROs)- an organization that is hired to outsource clinical trials or perform other clinical research support.
What qualifications or training do I need as a CPM?
CPM can come from different educational backgrounds and fields. Most have, at a minimum, a bachelor’s degree in a sciences field such as biology, health, life sciences or bioengineering. Those with advanced degrees such as MS, MBA, Ph.D. or MD will find it easier and faster to reach the CPM position, as long as they have relevant clinical trial and project management experience.
As a CPM, you will be working in a highly regulated environment and should have an expert understanding of Good Clinical Practice (ICH-GCP) and other relevant regulatory requirements. You should also have exposure working with institutional review boards (IRBs) or ethics committees (ECs).
Generally, a principle CRA with about 7-9 years of experience would be a strong candidate for a CPM position. A position as a clinical team lead, clinical team manager, or clinical operation leader would also serve as an excellent transition towards becoming a CPM. These positions offer valuable clinical leadership and management experience without some financial management responsibilities, such as budgeting.
Take courses from CCRPS and learn more on how to become a clinical research project manager.
Discover more from Clinical Research Training | Certified Clinical Research Professionals Course
2020 Clinical Research Internships
We provide clinical research internship which is follow the health policy related to clinical trials, research transparency. To know about the internship visit us now!
International Clinical Trials Registry Platform (ICTRP)
The International Clinical Trials Registry Platform (ICTRP) has a strong internship program which welcomes interested and enthusiastic students, professionals, and researchers from a wide range of disciplines. Internships with the ICTRP are flexible and tailored to individual learning goals to provide interns with the best possible experience with the ICTRP at the World Health Organization.
Internships with the ICTRP provide an excellent opportunity to participate in the work of health policy related to clinical trials, research transparency, and knowledge sharing.
Yale University – New Haven, CT
An internship that provides an opportunity to develop professional skills in a wide variety of fields and to support various university initiatives. Observes and gains expertise in applicable areas. Assists and completes various projects, programs, and assignments.
Essential Duties:
Working with and shadowing senior clinical research faculty in clinical research Human subject protection and regulatory training
Meeting regularly with faculty and receive mentoring
Working with regulatory and subject recruitment units
Rotations in inpatient and outpatient research clinics
Yale Center for Clinical Investigation Internship Program
Vanderbilt School of Medicine - Nashville, TN
The Vanderbilt Undergraduate Clinical Research Internship Program (UCRIP) offers college students earning a four-year degree the opportunity in research and clinical patient care at the academic medical center. This program is designed for students who are interested in a career in medicine.
Participants will design and complete research project from June to July, presenting results at the end of the program. The project may be in clinical or bench science research. Participants will be placed under the directorship of a research mentor. In addition, they will be spending time with residents and attending physicians.
Undergraduate Clinical Research Internship Program
NYU Langone Health - NYC, NY
This program is open to undergradute as well as post-baccalaureate students. The program is created by the Ronald O. Perelman Department of Emergency Medicine and consists of clinical and non-clinical research shifts based in the Emergency Department.
RA interns will:
develop basic research skills
be taught how to collect and enter data, and maintain databases
participate in laboratory meetings
gain Principal Investigator mentor-ship and/or shadowing experiences
RA interns will also become oriented to the Institutional Review Board and other Human Subjects courses
Take courses from CCRPS and learn more on how to become a clinical research professional.
Discover more from Clinical Research Training | Certified Clinical Research Professionals Course
Top 3 Clinical Research Blogs For Students and Professionals
WHAT IS A CLINICAL RESEARCH STUDY?
A clinical study is medical or drug research that involves research using human volunteers (also called participants). There are two main types of clinical studies: clinical trials (also called interventional studies) and observational studies.
Here’s a selection of the top clinical research blogs that will help you stay up-to-date with the latest industry trends, topics, and research breakthroughs:
Improving patient recruitment methods through the interpretation of actual clinical trial data is the then goal of Placebo Control, as you continue to read the posts in Placebo Control, you’re enlightened by Paul’s ideas, insights, and possibilities that could potentially reshape the industry.
I think the main thing that makes this blog unique is receiving direct insights from industry executives themselves. You’ll also see a lot of commentaries, reports, and arguments from experts
A blog that’s strictly professional. Dan Sfera originally started this blog to attract study participants for his research. What happened instead was totally unexpected. Surprisingly, Dan received a lot of comments and inquiries from clinical professionals instead of patients. Today, the blog caters to clinical professionals, and tips on how to survive the industry.
Take courses from CCRPS and learn more on how to become a clinical research professional.
Discover more from Clinical Research Training | Certified Clinical Research Professionals Course
Clinical Researchs Regulations
Clinical research regulations are set in place to make sure that all clinical research projects are conducted in accordance with international principles. The following regulations are the basics of the clinical research profession, and good for any aspiring professionals to know.
Good Manufacturing Practice (GMP)
This regulation is set in place to ensure that manufactures investigational medications and products to be safe and meet standards. This ensures that all participants involved in the clinical trials are not exposed to poor, harmful, and ineffective drugs. For those interested in further enhancing their knowledge and skills in this area, consider enrolling in the Advanced Clinical Research Project Manager Certification course.
Good Clinical Practice (GCP)
Good clinical practice is a major regulation that is set in place to conduct all clinical trials in accordance with the internationally recognized principles of the ICH GCP. It is there to ensure that risks to patients and volunteers are minimized. If you are looking to build a foundation in this critical area, our ICH GCP course can be an excellent resource.
Good Clinical Practice and Good Manufacturing Practice inspections and Enforcement
The MHRA checks the principles and standards of the GMP and GCP to ensure quality and that compliance are followed. They also help identify non-compliance and provide enforcement power. To learn more about roles in this regulatory field, explore our Medical Monitor Certification course.
Incapacitated Adult's Protection
This regulation is set in place to protect adults that are incapable of giving informed consent (e.g advanced Alzheimer's disease). The decision to consent to, refuse, or participate in a trial is made by an independent "consultee" that would act on the basis of the incapacitated adult wishes. Enhance your understanding of such roles with the Advanced Principal Investigator Physician Certification.
Minor's Protection
This regulation is set in place to protect minors (persons under the age of 16) and ensure that the following before a minor’s participation in trials:
REC consideration of receiving advice on the pediatric care relevant field.
A parent, custodian, guardian, or legal representative must give informed consent.
The minor should be informed of the risks and benefits of the trial according to their understanding capacity by staff experienced with young people.
The minor's wishes to be withdrawn from the trial or refusal to participate from the trial should be considered.
The clinical trial must be directly related to the illnesses the minor suffers from.
The clinical trial must directly benefit the minor involved.
To delve deeper into regulations protecting minors and their application in clinical trials, our Clinical Trials Assistant Training provides comprehensive insights.
Pharmacovigilance Arrangements
This regulation takes into account all the clinical trials of an IMP. It ensures that the reviews of adverse events and processing of serious adverse events are reported immediately to sponsors. In addition, it mandates that there must be a record of suspected unexpected serious adverse reactions caused by the trial medicines and that they must be reported to the MHRA. For those interested in specializing in this critical aspect of clinical trials, consider our Pharmacovigilance Certification.
If you want to learn more about the regulations of clinical research, take our free ICH GCP course. This course will help you build the knowledge for a solid foundation for a career in clinical research.
Take courses from CCRPS and learn more on how to become a clinical research professional.
Discover more from Clinical Research Training | Certified Clinical Research Professionals Course
The 4 Clinical Research Phases
Clinical researches are carried out in four phases to ensure consistency and accuracy.
Let's go through the four phases of clinical research.
Phase 1: Phase 1 starts with the first clinical trials performed on people. This phase immediately follows the preclinical research assessment of tests and treatments on animals. This shows how a drug is processed in the human body and its effects. A very small dose is administered to a group of 10 - 15 people. To learn more about the roles and responsibilities during this initial phase, consider enrolling in the Clinical Trials Assistant Training course.
Phase 2: This phase is tested out on a larger, yet still small group of 15 - 30 people. Low doses are given before they are increased till side effects become too severe or the desired effect is seen. Phase 2 tests the safety of the drugs before it can be moved to the next phase. For those interested in further exploring drug safety and its monitoring, the Pharmacovigilance Certification might be of interest.
Phase 3: This phase further assesses the safety and effectiveness of the drug. This time it is administered to 100 - 300 patients. New combination of treatment and drugs are tested and if found to effective, the new drugs are compared to the standard of care drug. They are compared to ascertain which drug works better. The trials are randomized. Patients are randomly placed into groups and tested with different treatments. This phase is quite important as it helps and provides the necessary information needed for the FDA to approve the new drug to be used in the general public. Aspiring Clinical Research Coordinators can deepen their understanding by taking the Clinical Research Coordinator course.
Phase 4: This final phase tests the newly FDA approved the drug in several hundred or thousands of patients. This enables better research and identification of short-lived and long-lasting side effects of the drugs. For professionals interested in leading clinical trials, the Advanced Clinical Research Project Manager Certification offers extensive training.
Generally, Phase 1 is the earliest phase of clinical research, but sometimes clinical researchers or clinical professionals might need you to join a phase 0 study. Phase 0 studies are aimed at finding out if a drug behaves the way it did in the laboratory studies. Phase 0 studies involve a small number of people (10 - 20) who are given a very small dose of the drug. The dose of the drug is so little that the chances of having any side effects are of the most minimal.
All these phrases are performed by clinical research scientists. They perform medical research for the main purpose of improving human health. They design studies on the investigation of particular diseases, the development of the medical device, and the evaluation of a drug's safety or effectiveness. They are also responsible for getting the funding for their experiments by periodically writing for grants and proposals for submission to governmental agencies and private organizations. Clinical research scientist can work anywhere, be it in the university, private organizations, hospitals, and research institutions. For physicians aiming to become principal investigators, the Advanced Principal Investigator Physician Certification and for those looking to specialize in medical monitoring, the Medical Monitor Certification are valuable resources.
Take courses from CCRPS and learn more on how to become a clinical research professional.
Discover more from Clinical Research Training | Certified Clinical Research Professionals Course
Paying Jobs for Pre-Med Students
When it comes to clinical research jobs for pre-med students, they are a bit hard to find. Luckily enough, we've got you covered. There are several pre-med jobs that are available to help you gain clinical research experience before you've completed your bachelor's degree. And yes, they are paying jobs.
These jobs will help showcase your interest and competence for any graduate program of your choice; be it dental school, physician assistant (PA), pharmacy, occupational therapy, medical school, or physical therapy (PT).
Emergency Medical Technicians
EMTs work for the emergency department or ambulance service in hospitals. They are specifically trained to manage and assess common medical emergencies from accidents and illnesses. They assist the nurses and doctors in the treatments of patients in the emergency rooms. Training requires 120 - 150 hours of instructions within 3 - 11 weeks. Consider advancing your skills with Advanced Clinical Research Project Manager Certification to further enhance your capabilities in clinical settings.
Phlebotomist
Phlebotomists work in blood banks and clinical research labs. They are trained in drawing blood from patients and are expected to complete 200 hours of instructions. To expand your career in clinical research, explore the Clinical Research Coordinator and Clinical Trials Assistant Training courses.
Certified Nursing Assistants
CNAs work in nursing homes, rehabilitation facilities, and hospitals. They help in assisting the nursing staff with acute care and long-term care. They are trained on how to control infections, and assists patients or residents with their daily routine. CNAs are in really high demand and the best way to become one is to have CNA experience, get certified through 4 - 15 weeks programs, and pass a state exam.
Dental Assistants
Dental Assistants work in dental offices. They are trained to maintain patient records, take x-rays, and provide patient care. They do not require certification. All you need to do is go through some formal training programs in dental offices.
Medical Scribes
Medical scribes work with physicians personally during patient visits and help documents all important information into the Electronic Health Record (EHR) of the patient. They are trained to organize information and understand medical terminologies. Enhance your understanding of good clinical practices with the ICH-GCP course.
Pharmacy Technicians
They work in clinical and retail pharmacies. They are there to assist the pharmacist with any technical tasks. The job prepares you for a career in pharmacy. Explore our Pharmacovigilance Certification to learn about ensuring the safety and efficacy of pharmaceutical products.
Even though you are a pre-med means, there are several salary earning pre-med jobs you can choose from. Make a wise choice today, join the CCRP community and let us help you build your career.
Take courses from CCRPS and learn more on how to become a clinical research professional.
Discover more from Clinical Research Training | Certified Clinical Research Professionals Course
How to Choose a Clinical Research Degree
Learn how to get a clinical research degree. Research the education requirements, training here. Visit now!
a clinical research degree makes you competent enough to function in the field of determining the safety, effectiveness, and efficacy of medical devices, new drugs, new treatments, and pharmaceutical products. There is no shortage of work for clinical research professionals as long as they have their degree at hand. The field is also a booming industry that is looking to raise the roof of medical advancement with competent certified clinical research professionals.
Government agencies like the FDA and many private companies are on the lookout for clinical researchers and managers that will monitor new treatment, medicines, and diagnostic products that are hitting the healthcare market every day.
Clinical research degrees include the following:
The expertise and involvement in the clinical field must be determined by the membership in the Consortium of academic programs in clinical research.
They must be the accessibility to students which be determined by the academic and professional experience required for admission.
Career training and curriculum relevance must be determined by the balance of technical and managerial coursework.
The application of knowledge type of experiential learning the requirements to graduate and significance to graduate must be instilled in the curriculum.
Students seeking a clinical research master's degree are sometimes already employed in the healthcare industry. The clinical research managers master's degree program offers plenty of flexibility in scheduling including online, offline, or hybrid course options.
For example, the University of Texas at Rio Grande Valley offers clinical research laboratory and hospital workers a convenient and affordable clinical research management master's degree program that is accelerated to be completed within 12 months. Its tuition is $1,008 per year. It is a top choice for working clinical research professionals who need to earn a paycheck while building their credentials. However, their accredited MS in clinical research is designed only for MDs.
On the other hand, the University of Texas Health Science Center at Houston is designed for clinicians and MD's at the faculty and fellow level. Its accredited MS in clinical research is a tough one that calls for a minimum of two years of courses delivered in weekly 90-minute lectures. Lectures are divided into the study design of clinical research, and emphatic genetics and molecular biology of translational research. Its tuition is $17,318 per year.
Degrees for clinical research sometimes has extreme strict admission requirements. So, it is important that each applicant consider each of their options carefully and choose the one that suits their needs and background best.
Take courses from CCRPS and learn more on how to become a clinical research professional.
Discover more from Clinical Research Training | Certified Clinical Research Professionals Course
Why Should You Take Clinical Research Training
Clinical Research training is a prerequisite for all certification as clinical research professionals. Clinical research training is committed to providing support to facilitate the highest possible quality in clinical research. CCRPs provides training opportunities for faculty and research staff to ensure that research is carried out efficiently and appropriately. It also takes into account the safety of Clinical research.
The clinical research training program includes professional development for the whole clinical research faculty, this includes; clinical research nurses, clinical research coordinators, clinical research data managers, clinical research regulatory officers, clinical research administrative officers, and clinical research financial staff. The training is for anyone who is a clinical research professional or is involved in clinical research.
Clinical research training can be done online or in institutions, and it is done to maintain broad-based information on the local state federal and institutional requirements that are needed to initiate and manage clinical research. It includes detailed knowledge about the specifics of implementing research concrete system for educating and updating those involved in clinical, translational research and also observational research about the compliance and regulatory requirements and procedures of the clinical research. The training program is also designed to help educate research staff on how to resolve problems encountered in their work environment.
Courses are open to all faculty and clinical research staff including the support staff. They include the following;
Good clinical practice training (ICH-GCP) also is known as GCP. GCP is very important as sponsors and are asking of it before initiating trials. GCP training twice a year and it's done face-to-face with experience, competent, and qualified GCP instructors
Introduction to a clinical trial. Although we at CCRPS do not offer clinical trials it is a prerequisite to understanding clinical research procedures.
CCRPs offers the lowest fees for online courses there are over 110 courses to choose from and all of them are for a little fee per year. With the online clinical research training from CCRPS, you can go on to establish a name for yourself in the clinical research industry. Clinical research managers and clinical research Associates (CRA) are the hotcakes in the clinical research industry presently. They are by far the most wanted clinical research professionals earning on an average nothing less than $55000 at least and over $120,000 per annum.
Make the smart choice today, switch to CCRPS take the online courses and upgrade your career.
Take courses from CCRPs and learn more on how to become a clinical research professional.
Discover more from Clinical Research Training | Certified Clinical Research Professionals Course.
What to Know About Working at Eurofins Clinical Research Laboratories (CRL)
A medical laboratory or clinical laboratory is a laboratory where clinical pathology tests are carried out on clinical specimens to obtain information about the health of a patient to aid in the diagnosis, treatment, and prevention of disease. Medical laboratories vary in size and complexity. They also offer different testing services.
Eurofins Clinical Research Laboratories (CRL) was established in 1992. Today, CRL operates as a contract laboratory providing a wide range of clinical safety and efficacy testing. Located in central New Jersey, CRL is dedicated to conducting human clinical test procedures to determine the safety and efficacy of cosmetic, personal care, and OTC drug products.
One of CRL’s specialties is providing human repeated insult patch testing (HRIPT) as both shared and exclusive panels. Various safety-in-use (SIU) studies are provided to meet the needs of any specific product category. There are different safety tests that CRL provides which can be modified to meet new and emerging technologies.
The staff of clinical laboratories may include:
Pathologist.
Clinical Biochemist.
Pathologists' Assistant (PA).
Biomedical Scientist (BMS) in the UK, Medical Laboratory Scientist (MT, MLS or CLS) in the US or Medical Laboratory Technologist in Canada.
Medical Laboratory Technician/Clinical Laboratory Technician (MLT or CLT in the US).
Medical Laboratory Assistant (MLA).
Phlebotomist (PBT).
Histotechnologist/Histology Technician.
What is a Clinical Laboratory Scientist?
The average pay for a Clinical Laboratory Scientist is $30.99 per hour.
Clinical laboratory scientists generally work for hospitals and independent medical laboratories. Candidates for this position are generally required to have a bachelor’s degree in biology, chemistry, laboratory science, or a related field, as well as necessary certifications as required by employers.
Eurofin CRL is a huge employer of clinical laboratory staff. If you are on the look out for a career in clinical research, check out their website here.
Additionally, for those interested in expanding their qualifications, consider the Advanced Clinical Research Project Manager Certification or the Advanced Principal Investigator Physician Certification offered by CCRPS to further your professional development.
Take courses from CCRPS and learn more on how to become a clinical research professional. Enhance your career by exploring specialized roles through comprehensive training like the Clinical Research Coordinator, Pharmacovigilance Certification, CRA Certification, ICH-GCP Training, and the Clinical Trials Assistant Training. These courses are designed to equip you with the necessary skills and certifications to excel in the field of clinical research.
For those involved in medical monitoring, the Medical Monitor Certification is a crucial resource for staying current with industry standards and regulatory requirements, ensuring that safety and efficacy tests align with professional expectations.
Discover more from Clinical Research Training | Certified Clinical Research Professionals Course
A Student's Guide to Clinical Certificate programs
This one-year certificate program, will let you learn research design concepts, analysis, and the quality assurance required for daily operations in the clinical research field. You will examine clinical studies across the pharmaceutical, medical device and natural health product sectors that give the competitive edge required in this dynamic industry. In this hands-on program, you will work alongside industry professionals and develop a network of contacts ahead of graduation.
The Clinical Research Certificate provides training in research methods for faculty members, postgraduate residents, international fellows and community-based health care professionals who want to improve their knowledge and skills in research methodology and quality improvement. For those interested in deepening their practical expertise, consider the Clinical Research Coordinator course from CCRPS.
Completion of the certificate will significantly enhance your ability to understand, effectively use, engage in and collaborate in research. It is not intended as a substitute for a research-based graduate degree, nor is it intended to lead to independent research projects (i.e., to take on a Principal Investigator role on large projects). For more intensive roles, the Advanced Principal Investigator Physician Certification might be suitable.
Work Experience
Optional Work Term
Students meeting all academic requirements may have the opportunity to complete an optional work term(s) in a formal work environment. The work term(s) is similar in length to an academic semester and typically involves full-time work hours that may be paid or unpaid. In programs with limited work term opportunities, additional academic requirements and a passing grade on a communication assessment may be required for eligibility. Eligibility for participation does not guarantee that a work position will be secured. Additional fees are required for those participating in the optional work term stream regardless of success in securing a work position.
Your Career
As a graduate of this program, you may pursue future career options, such as:
Clinical research associate (CRA)
Skills
Throughout this program you will develop the following skills:
Conduct research design concepts.
Analysis and quality assurance of daily operations in clinical trials development process for products such as drugs, medical devices, biopharmaceuticals and natural health products. For detailed training, explore the Pharmacovigilance Certification and ICH-GCP courses.
Identify the roles and responsibilities of the different positions within the clinical research processes.
Learn to incorporate ethical practices.
Program Objectives
After completing this certificate program, students will be better able to:
Construct meaningful research questions applicable to primary care research.
Compare and describe qualitative and quantitative research methodologies.
Design and contrast quantitative and qualitative data collection and analysis procedures
Appraise and critique data reported in the literature from research studies.
Identify, discuss and complete a grant proposal for submission to a research ethics board.
Clinicians seeking greater expertise and confidence in the use of research in their practice should consider the Medical Monitor Certification to further validate their credentials.
Applicants must be health care professionals licensed and in active practice in their country of primary residence and a Certificate of Completion will be issued by the Office of Continuing Professional Development to participants who satisfactorily complete the program.
Learners are evaluated on each component on a pass / fail basis, therefore there is no terminal examination or thesis and all components must be passed.
Take courses from CCRPS and learn more on how to become a clinical research professional.
Discover more from Clinical Research Training | Certified Clinical Research Professionals Course.
Clinical Research Courses for CTA and CRA
Clinical research courses are necessary to ensure that there are enough competent, responsible, and efficient clinical research professionals in the clinical research field. In this article, we'll be paying attention to the clinical research courses for Clinical Trial Assistants (CTAs), and Junior or Senior Clinical Research Associates (CRAs).
CTA
CTAs are one of the crucial role players in the clinical research process. They maintain the project's records, files, documentation and store them. They also assist with the review of clinical project records for accuracy and make them complete and ready for audit.
The CTA course offered at CCRPS are for those who are new to the job and want to cover the basics and principles of clinical research.
The program will highlight ICH GCP as the clinical research basis to introduce the new CTAs to drug development and to be familiar with the rules. It covers clinical studies operations, data management, and informatics, investigational product development and regulation, etc.
CRA
Junior Clinical Research Associates (CRAs) are introduced to Clinical Research, the life cycle of a clinical trial, how to monitor the clinical trial activities at different stages, clinical data monitoring, and management. It helps student become more familiar with the job of a CRA from the start of a clinical trial to its end. The course offered are similar to as those of a CTA’s.
Senior Clinical Research Associates (CRAs) are given opportunities to face more complex clinical trials and site management issues. The program will highlight the importance of international research as well as the role of the ICH GCP process. It also gives updates on the overview of current legislative requirements including:
guidance on substantial trial modifications,
improving the recruitment process of subjects and site staff
how to prioritize and upgrade clinical trial monitoring tasks and activities
accurate monitoring and reporting, dealings with non-compliance
efficient tracking and coaching and mentoring junior CRAs
The courses offered will expand the knowledge and improve the monitoring skills to help take and make the right actions and decisions to resolve site issues.
Each of the courses is designed to produce competent clinical research professionals.
Take courses from CCRPS and learn more on how to become Clinical Trial Assistants, and Junior and Senior Clinical Research Associates.
Take courses from CCRPS and learn more on how to become a clinical research professional.
Here are some courses you might consider:
Clinical Research Coordinator Certification - Ideal for those looking to coordinate clinical trial activities.
Pharmacovigilance Certification - Focuses on drug safety and adverse effects management.
CRA Certification - Prepares you for the role of Clinical Research Associate.
ICH-GCP Certification - Essential for professionals needing to understand Good Clinical Practice guidelines.
Clinical Trials Assistant Training - Provides foundational knowledge for those starting in clinical trials.
Advanced Clinical Research Project Manager Certification - For experienced professionals aiming for project management roles.
Advanced Principal Investigator Physician Certification - Tailored for physicians leading clinical trials.
Medical Monitor Certification - Designed for those overseeing the medical aspects of clinical trials.
Discover more from Clinical Research Training | Certified Clinical Research Professionals Course
What To Know About Clinical Trial Protocols
Clinical Trial Protocol
Download our advanced review of the: Clinical Trial Protocol PDF
What is a Clinical Trial Protocol
Clinical trial protocols are the plans that are followed by all clinical trial professionals. They are designed to balance the benefits and risks of all clinical trials. The clinical trials are often sponsored by clinical trial sponsors who are responsible for the managing, initiating, and/or financing of clinical trials, but they do not conduct or supervise the clinical researches. Clinical trial sponsors include people, companies, government agencies, organizations, or other institutions.
What is the purpose of a Clinical Trial Protocol
The protocols are guidelines to answer specific research questions. They include the following; the goal of the study, the details on tests, treatments, and procedures, the eligibility of participants that will take part in the clinical trials, the length of the trial, the information needed and gathered, and protections against the risks to participants.
A clinical trial is always led by a Principal Investigator (PI), who direct members of the clinical research team regularly monitor the health of the participants to help determine and evaluate the safety and effectiveness of the study.
There are different types of clinical trials to you can work in, from which you can earn $60,412 - $160,876 a year.
Screening Trials: This trial tests new ways of detecting diseases or health conditions.
Prevention of Trials: This trial looks for the best way to prevent diseases from happening or reoccurring.
Diagnostic Trials: This trial studies and compares tests or procedures in the diagnosis of a particular disease or condition.
Behavioral Trials: This trial evaluates and compares ways to promote behavioral changes to improve health
Supportive Care Trials: This trial explores the new ways to improve comfort and quality of life for people with illnesses and conditions.
Treatment of Trials: This trial tests new treatments, a new combination of drugs, new drugs, new surgical procedures, new radiation therapy, and new medical approaches.
There are four phases of clinical trials which serve different purposes and help answer different questions.
Trials Phase 1. This is the testing of drugs or treatments within a small group of people ranging from 20 - 80 to study the drugs or treatments to learn and identify the side effects and safety of the drugs.
Trials Phase 2. This is the testing of drugs or treatments within a large group of people ranging from 100 - 300 to determine the effectiveness of the drugs or treatments and to further study its safety.
Trials Phase 3. This is the testing of new drugs or treatments within a large group of people ranging from 1,000 - 3,000 to monitor its side effects, its effectiveness, it's standards, and also to collect information on how the new drugs or treatments can be used safely.
Trials Phase 4. This is the phase where the new drugs or treatments get approved by the FDA and is made available to the public, to track its safety and to also seek more information about the benefits of the drugs and the treatments.
Learn More
Take courses from CCRPS and learn more on how to become a clinical trial professional with our training programs:
Discover more from Clinical Research Training | Certified Clinical Research Professionals Course
Full List of CCRPS Clinical Research Courses
Clinical research courses are a prerequisite to any certification in the clinical research field. Clinical research is the investigation that is focused on determining the safety and efficiency of new medical products that includes; devices, diagnostic agents, vaccines, drugs, and treatment procedures that are intended to maintain, regain, and experiment new possibilities for human health.
Clinical research looks into the prevention, treatment, diagnosis, and relieving symptoms of diseases. They carry out medical investigations on healthy and diseased human volunteers to generate information that can be used to treat, diagnose, prevent, and curtail diseases and their symptoms. Clinical professionals develop, research, and test new applications in medical and clinical science, as well as develop new applications which include, medical procedures, vaccines, and pharmaceuticals to be tested in clinical trials.
So, what are the minimum requirements needed to study clinical research courses?
Doctorate degrees (M.B.B.S, B.S.S.M, B.A.M.S, B.V.Sc., B.H.M.S, B.D.S, B.U.M.S.)
Bachelor’s degree in Pharmacy or Physiotherapy (B.Pharm., M.Pharm.)
Life Sciences (Biochemistry, Toxicology, Biotechnology, Microbiology, Botany, Zoology, or Pharmacology)
Nursing
Taking these courses will open a lot of job opportunities in the clinical research field, as you can occupy any of the following posts;
Clinical Research Associate (CRAs)
Clinical Research Analysts (CRAn)
Biostatistician, etc.
Since clinical research deals with the diverse experiments conducted with living organisms or humans, the major parts of the course will focus on the following;
The organic activities performed in the clinical trial sites.
The study of drugs and medicine management.
The manufacture of medicine and drugs
The study of related disciplines such as biochemistry, epidemiology, biostatistics, transplantation, etc.
The advanced study of the sub-specialties of data management, epidemiology, biostatistics, and research design.
The determination of the appropriate quantity and quality of drugs to be ingested or taken into the body, and the impact of it to the body, as well as the reaction of the body to the drugs.
All candidates that are enrolled into the course are expected to choose an area of specialization from the following:
Genetics
Patient-specific research
Translational research
Since clinical research deals mainly with the research of medical equipment and medicines based on their safety and performance, it is one of the most important industries pertaining to the healthcare domain. Additionally, it is also one of the highest paying industries, with the average annual salary ranging from $56,984 - $156,678.
Take courses from CCRPS and learn more on how to become a clinical research professional.
Discover more from Clinical Research Training | Certified Clinical Research Professionals Course
International Educational Services
The curriculum and syllabus are important parts of any education and training programs for clinical research professionals. Thus, they help determine the structure and quality of international educational services around the world.
One of the foremost international educational services institutions is Barnett International. Barnett International was founded in 1979 and is widely known for its educational courses, training consulting services, and training programs for clinical research professionals all around the world.
Clinical research training platforms vary depending on the learning requirements. Barnett International offer training, interactive web training sessions, and e-learning modules for specific clinical research roles. They provide students with a training experience that is uniquely focused on core competency development. Their clinical training curriculum covers Clinical Research Coordinators (CRCs), Clinical Research Associates (CRAs), Principal Investigator (PIs), Clinical Data Managers, Regulatory Affairs, Clinical Quality Assurance, Clinical Project Managers, and other clinical research roles. Most importantly, heir courses provide the flexibility and engagement for every kind of student.
For those looking to specialize further, consider enhancing your skills with specific courses such as the Clinical Research Coordinator course or the CRA training offered by CCRPS, designed to meet the needs of individuals aiming at these roles.
We can take a look at another international educational service institution, which is the Institute of Clinical Research (ICR). ICR is perhaps the oldest international educational service provider for clinical researchers. They are known for providing high-quality training, support, and networking to the clinical research community.
How do they achieve all this? The ICR strives to support their members throughout all the stages of their careers by defining and refining standards for the clinical research industry. They do so by providing an interactive forum where the discussion of key issues that affect and impact clinical research takes place.
They also provide opportunities for learning and development to enhance the competence of clinical research professionals. Clinical professionals that know what they are doing boost the public confidence, outlook, and understanding of clinical research as well as promote a good relationship with other healthcare related professionals.
CCRP is another international educational services provider that is known for their clinical research online training for every student. Courses like Pharmacovigilance Certification and ICH-GCP training cater to those interested in ensuring safety and compliance in clinical trials. The Advanced Clinical Research Project Manager Certification and Advanced Principal Investigator Physician Certification are ideal for those looking to ascend to higher roles within the field. Furthermore, the Medical Monitor Certification offers specialized knowledge for overseeing clinical trial safety.
CCRP courses are accredited by the ACCRE and curated by real life professionals that know what information is most important for a student to learn. The training course are self-paced and online. That means you can take the course on your own schedule and learn how you learn best.
The goal of these international educational service providers is to ensure and support excellence in clinical research. By implementing competent members with the training, certification, and development tools, their student can work ethically, responsibly, and professionally anywhere in the world.
Become a part of clinical research professionals and see yourself earning $81,654 annually on average. Researching jobs positions near you can give you a better idea of what is available in your location. Want to get started? Check out our website and let us help you succeed.
Take courses from CCRPS and learn more on how to become a clinical research professional with our Clinical Trials Assistant Training.
Discover more from Clinical Research Training | Certified Clinical Research Professionals Course