What to Know About Working at Eurofins Clinical Research Laboratories (CRL)
A medical laboratory or clinical laboratory is a laboratory where clinical pathology tests are carried out on clinical specimens to obtain information about the health of a patient to aid in the diagnosis, treatment, and prevention of disease. Medical laboratories vary in size and complexity. They also offer different testing services.
Eurofins Clinical Research Laboratories (CRL) was established in 1992. Today, CRL operates as a contract laboratory providing a wide range of clinical safety and efficacy testing. Located in central New Jersey, CRL is dedicated to conducting human clinical test procedures to determine the safety and efficacy of cosmetic, personal care, and OTC drug products.
One of CRL’s specialties is providing human repeated insult patch testing (HRIPT) as both shared and exclusive panels. Various safety-in-use (SIU) studies are provided to meet the needs of any specific product category. There are different safety tests that CRL provides which can be modified to meet new and emerging technologies.
The staff of clinical laboratories may include:
Pathologist.
Clinical Biochemist.
Pathologists' Assistant (PA).
Biomedical Scientist (BMS) in the UK, Medical Laboratory Scientist (MT, MLS or CLS) in the US or Medical Laboratory Technologist in Canada.
Medical Laboratory Technician/Clinical Laboratory Technician (MLT or CLT in the US).
Medical Laboratory Assistant (MLA).
Phlebotomist (PBT).
Histotechnologist/Histology Technician.
What is a Clinical Laboratory Scientist?
The average pay for a Clinical Laboratory Scientist is $30.99 per hour.
Clinical laboratory scientists generally work for hospitals and independent medical laboratories. Candidates for this position are generally required to have a bachelor’s degree in biology, chemistry, laboratory science, or a related field, as well as necessary certifications as required by employers.
Eurofin CRL is a huge employer of clinical laboratory staff. If you are on the look out for a career in clinical research, check out their website here.
Additionally, for those interested in expanding their qualifications, consider the Advanced Clinical Research Project Manager Certification or the Advanced Principal Investigator Physician Certification offered by CCRPS to further your professional development.
Take courses from CCRPS and learn more on how to become a clinical research professional. Enhance your career by exploring specialized roles through comprehensive training like the Clinical Research Coordinator, Pharmacovigilance Certification, CRA Certification, ICH-GCP Training, and the Clinical Trials Assistant Training. These courses are designed to equip you with the necessary skills and certifications to excel in the field of clinical research.
For those involved in medical monitoring, the Medical Monitor Certification is a crucial resource for staying current with industry standards and regulatory requirements, ensuring that safety and efficacy tests align with professional expectations.
Discover more from Clinical Research Training | Certified Clinical Research Professionals Course
What is a Clinical Research Psychologist
Learn about what a clinical research psychology does, skills, salary, and how you can become one in the future.
Clinical psychology research is a specialization within clinical research. It is the study of behavioral and mental health. In many ways, it is as important to the nation's health and well being as medical research.
In the same way that medical scientists work to understand the prevention, genesis, and spread of various diseases, clinical research psychologists conduct rigorous psychological research studies to understand, prevent, and treat the psychological conditions as it applies to individuals, couples, families, cultures, and diverse communities.
Empirical results gathered from psychological research studies guide practitioners in developing effective interventions and techniques that clinical psychologists employ - proven, reliable results that improve lives, mend troubled relationships, manage addictions, and help manage and treat a variety of other mental health issues. Clinical psychology integrates science with practice and produces a field that encourages a robust, ongoing process of scientific discovery and clinical application.
Role
Clinical research psychologists integrate the science of psychology and the treatment of complex human problems with the intention of promoting change. The four main goals of psychology are to describe, explain, predict and control the behavior and mental processes of others. This approach allows clinical researchers to accomplish their goals for their psychological studies, which is to describe, explain, predict, and in some cases, influence processes or behaviors of the mind. The ultimate goal of scientific research in psychology is to illustrate behaviors and give details on why they take place.
Clinical psychologists work largely in health and social care settings including hospitals, health centers, community mental health teams, Child and Adolescent Mental Health Services (CAMHS) and social services. They often work as part of a team with other health professionals and practitioners.
Salary and Education
The mean annual salary of a clinical psychologist is about $69,000, however, those with doctoral degrees can earn salaries of $116,343 or more. This industry is highly stable and growing, as psychological research becomes more important to various other industries.
If you want to become a clinical research psychologist, you need a master’s or doctorate degree. In these graduate programs, you will be trained at how to navigate this large body of research. In addition, many clinical psychology students are able to make significant contributions to the field during their education by assisting in labs and learning valuable field knowledge.
Research in clinical psychology is vast, containing hundreds if not thousands of topics. By engaging in research, we are investigating new ways to understand the human mind, and developing solutions to enrich the lives of all others, many students create current and up-to-date with psychology research at universities and research labs across the world.
Take courses from CCRPS and learn more on how to become a clinical research professional.
Discover more from Clinical Research Training | Certified Clinical Research Professionals Course
What is a Clinical Research Director
A clinical research director works to provide leadership in clinical research settings. They help oversee the overall evaluation and development of drugs or healthcare solutions, programs designed to prevent or treat infectious disease, and more. In short, they are responsible for the management of the research side of a healthcare organization.
For those looking to excel in such roles, gaining specialized knowledge through courses like the Advanced Clinical Research Project Manager Certification and Advanced Principal Investigator Physician Certification can be essential.
The director of research is a management position within the development services function. Research directors supervise the research staff. They set departmental and individual employee goals and provide routine evaluations of progress toward these goals.
Job duties
Develop operating procedures and ensure that the entire clinical research team follows those procedures. For comprehensive training on clinical research standards, consider the ICH-GCP course.
Create good overall guidelines for clinical research and ensure that the guidelines are followed
Assist in procuring specimens and samples for research needs
Help balance budgets and oversee the management of the clinical research team
Ensure that all federal and state regulations are followed completely
Handle HR roles including overseeing the hiring process and the employee management process. Enhance your skills in clinical research management through the Clinical Research Coordinator course.
Present progress reports and strategies for upper-level management
Serve as a director over the work of those employees who work under you
In essence, the job is a management level position that focuses on directing, organization, and administrating a clinical research facility. It is a role that requires a number of skills and capabilities, but one that offers numerous rewards to those who can excel in the position.
Characteristics
There are abilities that will only be mastered through education, but strengths in the following personal areas will have a big impact on your ability to excel in the job:
Strong Communication Skills – As a director, you’ll end up having to discuss options with your employees as well as your superiors. You will also be meeting with policymakers. Good communication is vital in these scenarios.
Confidence – You’ll also need to have confidence in your own abilities in order to complete your duties without hesitation or worry.
Strong Leadership Skills – In general, you’ll need good leadership skills in order to thrive in the role. Develop these skills through targeted training like the Clinical Trials Assistant Training.
Nature of the Work
The work will take you from the lab to the office to the board room….Further your expertise and adaptability in the field with courses like the CRA course and Pharmacovigilance Certification. As a clinical research director, you’ll divide your time between overseeing the activities going on in a facility, as wells as managing information and reports in your office. In some instances, you may have to visit conferences and other public functions in order to further hone your skills or to make the connections needed to move your research facility into the future.
Average salaries in the field vary around $132,000. While the low end of the salary range is roughly $87,000, it is still well above the national salary average for other jobs. In short, the position is one that can offer incredible pay and plenty of other benefits as well.
Take courses from CCRPS and learn more on how to become a clinical research director. Explore our wide range of certifications, including Medical Monitor Certification to broaden your expertise.
Discover more from Clinical Research Training | Certified Clinical Research Professionals Course
A Student's Guide to Clinical Certificate programs
This one-year certificate program, will let you learn research design concepts, analysis, and the quality assurance required for daily operations in the clinical research field. You will examine clinical studies across the pharmaceutical, medical device and natural health product sectors that give the competitive edge required in this dynamic industry. In this hands-on program, you will work alongside industry professionals and develop a network of contacts ahead of graduation.
The Clinical Research Certificate provides training in research methods for faculty members, postgraduate residents, international fellows and community-based health care professionals who want to improve their knowledge and skills in research methodology and quality improvement. For those interested in deepening their practical expertise, consider the Clinical Research Coordinator course from CCRPS.
Completion of the certificate will significantly enhance your ability to understand, effectively use, engage in and collaborate in research. It is not intended as a substitute for a research-based graduate degree, nor is it intended to lead to independent research projects (i.e., to take on a Principal Investigator role on large projects). For more intensive roles, the Advanced Principal Investigator Physician Certification might be suitable.
Work Experience
Optional Work Term
Students meeting all academic requirements may have the opportunity to complete an optional work term(s) in a formal work environment. The work term(s) is similar in length to an academic semester and typically involves full-time work hours that may be paid or unpaid. In programs with limited work term opportunities, additional academic requirements and a passing grade on a communication assessment may be required for eligibility. Eligibility for participation does not guarantee that a work position will be secured. Additional fees are required for those participating in the optional work term stream regardless of success in securing a work position.
Your Career
As a graduate of this program, you may pursue future career options, such as:
Clinical research associate (CRA)
Skills
Throughout this program you will develop the following skills:
Conduct research design concepts.
Analysis and quality assurance of daily operations in clinical trials development process for products such as drugs, medical devices, biopharmaceuticals and natural health products. For detailed training, explore the Pharmacovigilance Certification and ICH-GCP courses.
Identify the roles and responsibilities of the different positions within the clinical research processes.
Learn to incorporate ethical practices.
Program Objectives
After completing this certificate program, students will be better able to:
Construct meaningful research questions applicable to primary care research.
Compare and describe qualitative and quantitative research methodologies.
Design and contrast quantitative and qualitative data collection and analysis procedures
Appraise and critique data reported in the literature from research studies.
Identify, discuss and complete a grant proposal for submission to a research ethics board.
Clinicians seeking greater expertise and confidence in the use of research in their practice should consider the Medical Monitor Certification to further validate their credentials.
Applicants must be health care professionals licensed and in active practice in their country of primary residence and a Certificate of Completion will be issued by the Office of Continuing Professional Development to participants who satisfactorily complete the program.
Learners are evaluated on each component on a pass / fail basis, therefore there is no terminal examination or thesis and all components must be passed.
Take courses from CCRPS and learn more on how to become a clinical research professional.
Discover more from Clinical Research Training | Certified Clinical Research Professionals Course.
How to Find an International Clinical Research Job
Have you ever wanted to live in another country? International clinical research organizations (CROs) are always on the lookout for prospective clinical research professionals to fill the extensive range of positions that abound across the world in different company locations, sizes, and capacities.
The international scene posts new clinical research vacancies and job opportunities daily, and these clinical research vacancies and job opportunities ranges from management roles such as Study Manager (SM), Clinical Project Managers (CPMs), Clinical Trials Managers (CTMs), Clinical Research Coordinators (CRCs), Clinical Trial Assistants (CTAs), Clinical Research Associates (CRAs), and Clinical Directors (CDs) and Physical Investigators (PI) at global, regional, or local levels.
Let's take a look at some of the most popular international clinical research professional jobs on offer right now (as of May, 2019).
Clinical Project Manager: RBW Consulting Services located in the United Kingdom. Salary £55,000 - £65,000.
Clinical Project Manager: Cobham company in the United Kingdom. Salary £60,000 - £79,999.
Clinical Project Manager: Piper Company in the United States. Salary $125,000 - $140,000.
Clinical Trial Manager: PRA Health Services in the United States. Salary $117,000 - $171,000.
Clinical Director: United States Department of Health and Human Services. Salary $137,849 - $262,000.
The opportunities are diverse. You find find work ranging from medical technology, to pharmaceutical companies, to contract research organizations. A competent clinical research professional can find opportunities to work on various clinical trial projects in multiple companies.
No one will pay for half-baked professionals. Therefore, you have to develop yourself by taking courses from CCRPS and learn more on how to become a successful clinical research professional with these specialized courses:
Take courses from CCRPS and learn more on how to become a clinical research professional.
Discover more from Clinical Research Training | Certified Clinical Research Professionals Course
Clinical Research Courses for CTA and CRA
Clinical research courses are necessary to ensure that there are enough competent, responsible, and efficient clinical research professionals in the clinical research field. In this article, we'll be paying attention to the clinical research courses for Clinical Trial Assistants (CTAs), and Junior or Senior Clinical Research Associates (CRAs).
CTA
CTAs are one of the crucial role players in the clinical research process. They maintain the project's records, files, documentation and store them. They also assist with the review of clinical project records for accuracy and make them complete and ready for audit.
The CTA course offered at CCRPS are for those who are new to the job and want to cover the basics and principles of clinical research.
The program will highlight ICH GCP as the clinical research basis to introduce the new CTAs to drug development and to be familiar with the rules. It covers clinical studies operations, data management, and informatics, investigational product development and regulation, etc.
CRA
Junior Clinical Research Associates (CRAs) are introduced to Clinical Research, the life cycle of a clinical trial, how to monitor the clinical trial activities at different stages, clinical data monitoring, and management. It helps student become more familiar with the job of a CRA from the start of a clinical trial to its end. The course offered are similar to as those of a CTA’s.
Senior Clinical Research Associates (CRAs) are given opportunities to face more complex clinical trials and site management issues. The program will highlight the importance of international research as well as the role of the ICH GCP process. It also gives updates on the overview of current legislative requirements including:
guidance on substantial trial modifications,
improving the recruitment process of subjects and site staff
how to prioritize and upgrade clinical trial monitoring tasks and activities
accurate monitoring and reporting, dealings with non-compliance
efficient tracking and coaching and mentoring junior CRAs
The courses offered will expand the knowledge and improve the monitoring skills to help take and make the right actions and decisions to resolve site issues.
Each of the courses is designed to produce competent clinical research professionals.
Take courses from CCRPS and learn more on how to become Clinical Trial Assistants, and Junior and Senior Clinical Research Associates.
Take courses from CCRPS and learn more on how to become a clinical research professional.
Here are some courses you might consider:
Clinical Research Coordinator Certification - Ideal for those looking to coordinate clinical trial activities.
Pharmacovigilance Certification - Focuses on drug safety and adverse effects management.
CRA Certification - Prepares you for the role of Clinical Research Associate.
ICH-GCP Certification - Essential for professionals needing to understand Good Clinical Practice guidelines.
Clinical Trials Assistant Training - Provides foundational knowledge for those starting in clinical trials.
Advanced Clinical Research Project Manager Certification - For experienced professionals aiming for project management roles.
Advanced Principal Investigator Physician Certification - Tailored for physicians leading clinical trials.
Medical Monitor Certification - Designed for those overseeing the medical aspects of clinical trials.
Discover more from Clinical Research Training | Certified Clinical Research Professionals Course
What To Know About Clinical Trial Protocols
Clinical Trial Protocol
Download our advanced review of the: Clinical Trial Protocol PDF
What is a Clinical Trial Protocol
Clinical trial protocols are the plans that are followed by all clinical trial professionals. They are designed to balance the benefits and risks of all clinical trials. The clinical trials are often sponsored by clinical trial sponsors who are responsible for the managing, initiating, and/or financing of clinical trials, but they do not conduct or supervise the clinical researches. Clinical trial sponsors include people, companies, government agencies, organizations, or other institutions.
What is the purpose of a Clinical Trial Protocol
The protocols are guidelines to answer specific research questions. They include the following; the goal of the study, the details on tests, treatments, and procedures, the eligibility of participants that will take part in the clinical trials, the length of the trial, the information needed and gathered, and protections against the risks to participants.
A clinical trial is always led by a Principal Investigator (PI), who direct members of the clinical research team regularly monitor the health of the participants to help determine and evaluate the safety and effectiveness of the study.
There are different types of clinical trials to you can work in, from which you can earn $60,412 - $160,876 a year.
Screening Trials: This trial tests new ways of detecting diseases or health conditions.
Prevention of Trials: This trial looks for the best way to prevent diseases from happening or reoccurring.
Diagnostic Trials: This trial studies and compares tests or procedures in the diagnosis of a particular disease or condition.
Behavioral Trials: This trial evaluates and compares ways to promote behavioral changes to improve health
Supportive Care Trials: This trial explores the new ways to improve comfort and quality of life for people with illnesses and conditions.
Treatment of Trials: This trial tests new treatments, a new combination of drugs, new drugs, new surgical procedures, new radiation therapy, and new medical approaches.
There are four phases of clinical trials which serve different purposes and help answer different questions.
Trials Phase 1. This is the testing of drugs or treatments within a small group of people ranging from 20 - 80 to study the drugs or treatments to learn and identify the side effects and safety of the drugs.
Trials Phase 2. This is the testing of drugs or treatments within a large group of people ranging from 100 - 300 to determine the effectiveness of the drugs or treatments and to further study its safety.
Trials Phase 3. This is the testing of new drugs or treatments within a large group of people ranging from 1,000 - 3,000 to monitor its side effects, its effectiveness, it's standards, and also to collect information on how the new drugs or treatments can be used safely.
Trials Phase 4. This is the phase where the new drugs or treatments get approved by the FDA and is made available to the public, to track its safety and to also seek more information about the benefits of the drugs and the treatments.
Learn More
Take courses from CCRPS and learn more on how to become a clinical trial professional with our training programs:
Discover more from Clinical Research Training | Certified Clinical Research Professionals Course
Job Prospects in Clinical Trial
Clinical trials are research studies in which is include volunteers, doctors, and clinical researchers working together to learn more about diseases and how to improve health care for people.
A clinical trial is a branch of clinical research, which is a major component of medical research and it involves everyone including you. It consists of volunteers, doctors, and clinical researchers working together to learn more about diseases and how to improve health care for people.
Clinical trial includes the following branches:
Behavioral: This branch improves the understanding of human behavior and how it relates to diseases and health. Enhance your expertise in this area with the Clinical Trials Assistant Training.
Health Services: This branch focuses on the way people access health care services and service providers, how much health care costs, and the results of the care to the patients. Develop a deeper understanding by exploring our Advanced Clinical Research Project Manager Certification.
Epidemiology: This branch focuses on improving the understanding of a disease by studying, the cause, patterns, symptoms, effects, prevention, and cures in specific groups. Our Pharmacovigilance Certification can provide you with the tools to better analyze these patterns.
Clinical Trials: This branch helps evaluate the effects of an intervention on health outcomes. Get trained as a Clinical Research Coordinator to oversee these critical evaluations.
You must be wondering why you should be a part of the professionals that engage in clinical trials, right? Let's give you some insider scoop. Clinical trials are at the heart of all medical advancement around the world, and it provides one of the highest pay in all the medical field. Salaries range from $65,854 - $160,654. Clinical trials is important. It looks at new ways to detect, prevent, and treat diseases. Professionals study new medical devices, new drugs, new combinations of drugs, new surgical procedures, new ways to use existing treatments, new ways of behavioral changes to improve health, all to find new ways to improve the quality of life.
The main goal of all clinical trials is to determine if the detection, treatment, prevention, experimentation, and behavioral approaches to health care are effective and safe. Clinical trials offer hope for people with illness, diseases, or healthy volunteers receive new treatments and care as well as take part in finding new ways to help others.
Consider enhancing your capabilities with our CRA (Clinical Research Associate) course, ICH-GCP training, or the Advanced Principal Investigator Physician Certification to learn more about overseeing medical research studies. Additionally, for those interested in overseeing drug and device safety, our Medical Monitor Certification is available.
Take courses from CCRPS and learn more on how to become a clinical research professional.
Discover more from Clinical Research Training | Certified Clinical Research Professionals Course
Full List of CCRPS Clinical Research Courses
Clinical research courses are a prerequisite to any certification in the clinical research field. Clinical research is the investigation that is focused on determining the safety and efficiency of new medical products that includes; devices, diagnostic agents, vaccines, drugs, and treatment procedures that are intended to maintain, regain, and experiment new possibilities for human health.
Clinical research looks into the prevention, treatment, diagnosis, and relieving symptoms of diseases. They carry out medical investigations on healthy and diseased human volunteers to generate information that can be used to treat, diagnose, prevent, and curtail diseases and their symptoms. Clinical professionals develop, research, and test new applications in medical and clinical science, as well as develop new applications which include, medical procedures, vaccines, and pharmaceuticals to be tested in clinical trials.
So, what are the minimum requirements needed to study clinical research courses?
Doctorate degrees (M.B.B.S, B.S.S.M, B.A.M.S, B.V.Sc., B.H.M.S, B.D.S, B.U.M.S.)
Bachelor’s degree in Pharmacy or Physiotherapy (B.Pharm., M.Pharm.)
Life Sciences (Biochemistry, Toxicology, Biotechnology, Microbiology, Botany, Zoology, or Pharmacology)
Nursing
Taking these courses will open a lot of job opportunities in the clinical research field, as you can occupy any of the following posts;
Clinical Research Associate (CRAs)
Clinical Research Analysts (CRAn)
Biostatistician, etc.
Since clinical research deals with the diverse experiments conducted with living organisms or humans, the major parts of the course will focus on the following;
The organic activities performed in the clinical trial sites.
The study of drugs and medicine management.
The manufacture of medicine and drugs
The study of related disciplines such as biochemistry, epidemiology, biostatistics, transplantation, etc.
The advanced study of the sub-specialties of data management, epidemiology, biostatistics, and research design.
The determination of the appropriate quantity and quality of drugs to be ingested or taken into the body, and the impact of it to the body, as well as the reaction of the body to the drugs.
All candidates that are enrolled into the course are expected to choose an area of specialization from the following:
Genetics
Patient-specific research
Translational research
Since clinical research deals mainly with the research of medical equipment and medicines based on their safety and performance, it is one of the most important industries pertaining to the healthcare domain. Additionally, it is also one of the highest paying industries, with the average annual salary ranging from $56,984 - $156,678.
Take courses from CCRPS and learn more on how to become a clinical research professional.
Discover more from Clinical Research Training | Certified Clinical Research Professionals Course
What is a Clinical Research Analyst
In the wide field of clinical research professionals, there are lots of job opportunities as well as job posts. They include; clinical research coordinators, clinical research project managers, clinical research trial managers, principal investigators, clinical research analysts, and many more. In this article, we will talk about the clinical research analyst position.
What does a clinical research analyst job entail?
Clinical research is often conducted and carried out in clinics, hospitals, clinical research trial sites, medical facilities, or laboratories (private and individual companies or pharmaceuticals). In effect, clinical research analyst work hand in hand with clinical research professionals within the medical field. They work on medical studies designed to measure the effectiveness of a medical device, drug, or medical process on the human body.
A clinical research analyst directly works under clinical research management. They work within teams that conduct medical studies, clinical research, and clinical trials.
Clinical research analyst are one of the many key players in clinical trials and medical studies. They combine clinical sciences, scientific research, medical education, analytical and communications skills, and sometimes financial skills and knowledge to their job. Their role is very flexible. They can be in labs, directly working with patients, or they can be providing scientific or financial insight to physicians and principal investigators.
The general job description of a clinical research analyst includes the following;
Working with scientists or physicians who oversee the clinical research process and procedures.
Direct interaction with patients by interviewing and screening them as potential candidates.
Collecting data from the clinical trial sites for further and accurate study.
Carry out research accounting and budgeting, this is where their financial acumen comes into play.
Acting as liaisons between supervising physician investigators and other medical staff such as nurses.
Coordinating clinical research studies by tracking inventory, collation, and collection of data, identifying and interacting with patients, and overseeing protocols.
Acting as the primary financial resource to go to for clinical study.
To be a clinical research analyst you need to have a minimum of an associate’s degree for an entry-level qualification. You will need a bachelor’s or master’s degree for higher level positions. For a clinical research analyst, work experience in a clinical research setting or other clinical related field is a requirement.
The prospective job growth is promising, as it is projected at an estimated 16% growth till 2024, with an estimated annual salary of $60,520 and above.
Take courses from CCRPS and learn more on how to become a clinical research professional.
Discover more from Clinical Research Training | Certified Clinical Research Professionals Course
How to Become a Clinical Trial Manager
Clinical trial managers are one of the most important roles when it comes to the development of new medicines. They ensure the safety of the human subjects that are involved in a clinical trial. Apart from that, they also weigh and validate the outcomes of the trial against industry standards. Do you like the idea of being a clinical trial manager? Then here’s how to become a clinical trial manager.
The steps are quite simple. In preparing to become a clinical trial manager, you should obtain a bachelor's or master's degree in clinical sciences, life sciences, or other health sciences related fields. Get a post-baccalaureate certificate from CCRPS, and get administered by the Academy of Clinical Research Professionals.
A good clinical trial manager course should include enrollment into at least 6 required courses (site management, good clinical practices (ICH-GCP), drug development procedures, clinical research writing, and statistical analysis) and 1 elective to earn your clinical trial manager certification. You are also required to take some computer science courses to have knowledge of Clinical Trial Management System and Oracle Clinic, programs you will need to know for the job.
This job entails receiving accurate information, decision making, and problem-solving organizing skills. This means that you must be familiar with planning, work updates, establishing interpersonal relationship skills, and maintaining interpersonal relationships.
As a clinical trial manager you tend to be a working manager for smaller staff (this makes you a part of the research process). However, you could be a working manager with larger staff (this makes you stay in a supervisory role). Whatever the case might be, CTMs coordinate the activities of their unit with other units and makes sure that the financial, production, and marketing specialist are fully equipped with supplies, equipment, and materials. They are in charge of directing the activities related to the research, development, and coordination of clinical trials.
As a CTM you will:
hire, supervise, and evaluate technicians, staff members, and scientists
ensure that the laboratories are fully stocked and equipped with supplies and equipment
provide technical support to scientists and technicians
monitor the progress of projects
draft out operational reports
review all performed research and
report all researched results accurately
establish and make sure that administrative procedures, standard, and policies, are duly complied to and followed
direct and coordinate the development of project products, production activities, and scientific research activities (improving the process of manufacturing)
The average salary earned is between the range of $100,289 - $150,874, though that may vary based on location. Job boards are a great way to check on positions available near you.
Get a course at CCRP and get certified to become a Certified Clinical Trial Manager.
Discover more from Clinical Research Training | Certified Clinical Research Professionals Course.
International Educational Services
The curriculum and syllabus are important parts of any education and training programs for clinical research professionals. Thus, they help determine the structure and quality of international educational services around the world.
One of the foremost international educational services institutions is Barnett International. Barnett International was founded in 1979 and is widely known for its educational courses, training consulting services, and training programs for clinical research professionals all around the world.
Clinical research training platforms vary depending on the learning requirements. Barnett International offer training, interactive web training sessions, and e-learning modules for specific clinical research roles. They provide students with a training experience that is uniquely focused on core competency development. Their clinical training curriculum covers Clinical Research Coordinators (CRCs), Clinical Research Associates (CRAs), Principal Investigator (PIs), Clinical Data Managers, Regulatory Affairs, Clinical Quality Assurance, Clinical Project Managers, and other clinical research roles. Most importantly, heir courses provide the flexibility and engagement for every kind of student.
For those looking to specialize further, consider enhancing your skills with specific courses such as the Clinical Research Coordinator course or the CRA training offered by CCRPS, designed to meet the needs of individuals aiming at these roles.
We can take a look at another international educational service institution, which is the Institute of Clinical Research (ICR). ICR is perhaps the oldest international educational service provider for clinical researchers. They are known for providing high-quality training, support, and networking to the clinical research community.
How do they achieve all this? The ICR strives to support their members throughout all the stages of their careers by defining and refining standards for the clinical research industry. They do so by providing an interactive forum where the discussion of key issues that affect and impact clinical research takes place.
They also provide opportunities for learning and development to enhance the competence of clinical research professionals. Clinical professionals that know what they are doing boost the public confidence, outlook, and understanding of clinical research as well as promote a good relationship with other healthcare related professionals.
CCRP is another international educational services provider that is known for their clinical research online training for every student. Courses like Pharmacovigilance Certification and ICH-GCP training cater to those interested in ensuring safety and compliance in clinical trials. The Advanced Clinical Research Project Manager Certification and Advanced Principal Investigator Physician Certification are ideal for those looking to ascend to higher roles within the field. Furthermore, the Medical Monitor Certification offers specialized knowledge for overseeing clinical trial safety.
CCRP courses are accredited by the ACCRE and curated by real life professionals that know what information is most important for a student to learn. The training course are self-paced and online. That means you can take the course on your own schedule and learn how you learn best.
The goal of these international educational service providers is to ensure and support excellence in clinical research. By implementing competent members with the training, certification, and development tools, their student can work ethically, responsibly, and professionally anywhere in the world.
Become a part of clinical research professionals and see yourself earning $81,654 annually on average. Researching jobs positions near you can give you a better idea of what is available in your location. Want to get started? Check out our website and let us help you succeed.
Take courses from CCRPS and learn more on how to become a clinical research professional with our Clinical Trials Assistant Training.
Discover more from Clinical Research Training | Certified Clinical Research Professionals Course
Why Are Clinical Research Seminars Important
The purpose of the clinical research seminars is to impact the attendees with knowledge about important aspects of clinical research, such as the professional ethics, operational aspect and regulations. Education and training are very important in this field and vital to the success of trials. Seminars are another way of training clinical research professionals or other related professionals that have an interest in the field.
Seminars can be held every two weeks, monthly or quarterly. It depends totally on the organizers and what they aim to achieve with the seminar. For example, some organizers may hold a biweekly seminar for young interns to help them improve their knowledge and sharpen their clinical research skills.
Clinical research seminars are ideal for the following sets of people:
Nurses
Regulatory experts
Other staff also involved in clinical research can as well attend the seminar to improve their knowledge. Common topics that are discussed at the seminars include :
Professional ethics of clinical researchers.
Auditing clinical trials
Advanced quality monitoring
Monitoring oncology trials
Current regulations
Inspection preparation
Resources involved with clinical research.
Guidelines pertaining to clinical research.
These seminars are usually handled by experts and professionals in the clinical research industry. They are usually have worked with different leading pharmaceutical companies biotechnological companies, device companies, and leading academic institutions.
Clinical research seminars can also provide extra benefits. This depends a lot on who the organizers are, but some clinical research seminars provide those in attendance with SOCRA (Society of Clinical Research Associates), ACRP (Association of Clinical Research Professionals) and CME Continuing Education Credit hours.
Apart from seminars organized by different institutions that an individual can apply for, seminars can also be organized for in-house training. They can be tailored to meet your company's specific needs. Organizers can provide your company with a set of training options that range from single topic seminar presentation to more comprehensive programs. For instance, some organizers make use of different hands on approach by providing activities and simulated case studies to help the audience's learning process.
As an individual you can register yourself to become a participant of upcoming clinical research seminars at ccrps.org. As an organization or company as well, you can register your staffs for these seminars. As a company, you can register for in-house training, where the seminars can be tailored for your specific needs.
Take courses from CCRPS and learn more on how to become a clinical research professional.
Discover more from Clinical Research Training | Certified Clinical Research Professionals Course.
Free Online Clinical Research Courses
Clinical research plays a very vital role in medical health care. Before anything is put into practice, it will have to be discovered first and tested: this is the role of clinical research.
Free online courses on clinical research teach the basics of clinical research. They help students understand more about modern health care and the role of clinical research and its discovery. Courses like these are ideal for college students and people who are considering taking up a career in health care and life sciences. It is also good for social care practitioners that want to find out more information about the role of clinical research in improving the quality of healthcare.
Clinical research training for research professionals include taking necessary courses and getting the necessary accompanying certification. However, clinical research professionals can also take part in courses teaching the rudiments of clinical research and good clinical practice. An example of one of such courses is the one offered by National Institutes of Health (NIH). The course "Introduction to the principles and practice of clinical research" is a course that teaches professionals how to improve their effectiveness and to as well safety. This course covers topics like study design, monitoring and regulatory considerations, how to prepare and implement clinical studies, statistics, ethical and legal considerations in clinical research, measurements, additional study design etc. A course like this helps you to understand the very basics of clinical research and improves your efficiency.
As good as free online clinical research courses are to help you develop your career as a clinical research professional, they are not meant to take the place of the required training needed, especially those that train you in the protection of the human subjects of clinical research. For those interested in specializing further, consider the Advanced Clinical Research Project Manager Certification or the Advanced Principal Investigator Physician Certification.
For further details and information on how to get free online courses for clinical research professionals, visit ccrps.org. Whether you are a research professional looking to learn more and better your career or you are a student or someone just taking up an interest in the field of clinical research, there will be something for you.
Take courses from CCRPS and learn more on how to become a clinical research professional, such as the Clinical Research Coordinator, Pharmacovigilance Certification, CRA, ICH-GCP, Clinical Trials Assistant Training, or Medical Monitor Certification.
Discover more from Clinical Research Training | Certified Clinical Research Professionals Course
Online Clinical Research Assoicate (CRA) Training
Clinical research associate training is the grooming of clinical researchers to become associates. It's a promising career in the clinical research industry. Any worth-while training will include everything you need to know to about the job and become one of the best.
Clinical Research Associates are those responsible for the planning, setting up, and coordinating clinical trials. They provide technical assistance for experiments, make sure that the scientists that are involved in the clinical research trials comply with regulatory standards, and also collect results during the clinical trials. When the trials are over they are also to be involved in the presentation of the results to the public in a manner that is useful and understandable. Enhance your skills as a Clinical Research Associate with CRA training.
The amazing part about being a clinical research associate is that jobs are available in both the laboratory and office setting.
To be an associate in clinical research you need a minimum degree of a bachelor's or master's degree in the clinical research field of study or a related field. They have the key responsibilities of designing and implementing clinical research trials, to train staff, to monitor progress, to screen test subjects, to present findings, and to help maintain the database of all clinical research trainees.
The median salary of a clinical research associate boasts of $61,932 annually. It is one of the most attractive salaries for someone with a science degree, as it is always on a rise and there is always a ready market for clinical research professionals.
A clinical trial is a method in which new medical treatments and pharmaceuticals are developed and experimented and this is where clinical research associates come in. They are an integral part of the process, as they work with teams to design, implement, and organize research trials to test out the effectiveness of the proposed medicines on humans. They are responsible for the different parts of clinical trials which include, training of staff, screening test subjects, monitoring the progress of staff, present findings, and maintain the database. Communication skills, science knowledge, and a strong mathematics knowledge base are the core skills you need in the clinical research profession. For those interested in more specialized roles, consider the Advanced Clinical Research Project Manager Certification or the Advanced Principal Investigator Physician Certification.
You can come from a variety of medical sciences or health-related fields, such as a nursing background as an RN. Courses offered expand on clinical related ethics, team management, and research methodologies.
CRA training certificate examination comes with one of the three combinations of education and experience.
High school diploma and 6,000 hours of experience.
An associate degree in clinical research fields and 4,500 hours of experience, or
A Bachelor's, Master's, or Registered Nurse degree and 3,000 hours of clinical research experience.
Get a clinical research associate certificate from certified clinical research professionals course, and earn your way to one of the most lucrative fields in clinical research. At CCRPS, we support online learning, so you can take the classroom everywhere. Learn more about Clinical Research Coordinator training and Pharmacovigilance Certification to broaden your expertise.
Take courses from CCRPS and learn more on how to become a clinical research professional. Further your understanding of good clinical practices with the ICH-GCP course.
Discover more from Clinical Research Training | Certified Clinical Research Professionals Course
Clinical Research Coordinator Certification
Clinical research coordinator (CRC) play an integral role in all kinds of medical studies. Working under the direction of the principal investigator, who designs, conducts, and manages clinical trial; the clinical research coordinator supports, organizes, and facilitates the daily activities of the clinical trial.
A Clinical research coordinator manages, oversees, executes tasks and day to day clinical trial activities. They also work in conjunction with sponsors, departments, and institutions to manage finances, obtain compliance, and work through personal issues.
Their responsibilities include the planning and management of the following initiatives:
study
enrollment
maintenance
training
compliance with institutional, state, and federal regulations
CRCs engage directly with the trial participants, screening them for eligibility. They also help develop and implement recruitment strategies and work with all the available teams during the trial period.
In the lab, CRCs carry our experiments, medical studies, and clinical researches. A CRC works closely with labs, medical centers, and research hospitals to evaluate research protocols and seek approval from regulatory committees.
To get certified as a CRC, you must have either:
A bachelor's or higher degree in health sciences in clinical research administration and submission of a fully detailed resume that documents at least 3,000 hours of performed designated duties
A Registered Nurse (RN), LVN, Licensed Practical Nurse (LPN), or an associate degree and a submission of a detailed report that documents at least 4,500 hours of performed designated duties.
Worked as a laboratory technician, medical assistant, or a high school diploma and submission of a detailed report that documents at least 6,000 hours of performed designated duties.
For those with a bachelor’s, a degree in medical technology, public health administration, or microbiology, with an additional two years master's degree in management positions (optional) is ideals. Human anatomy, biostatistics, epidemiology, health care management, biochemistry, and mathematics, are the courses typically required for these majors.
Management and communications experience coupled with interpersonal and multitasking skills are essential job skills to possess. Internships and/or low entry-level jobs as health care workers or lab technicians should also be considered to get familiarized with the working environment. It is important to note that there may be a substitution for hours of qualifying work experience.
After qualifying, you need to pass an exam and get certified. A CRC certification lets you practice legally and can see you earning from as little as $48,000 to as much as $60,000 annually.
To learn more, take a course in clinical research coordination to help develop and refine your skills before you go on to get the clinical research coordinator certification.
Discover more from Clinical Research Training | Certified Clinical Research Professionals Course. Other recommended courses for aspiring professionals include:
These courses provide essential knowledge and training for those interested in advancing their careers in clinical research.
Take courses from CCRPS and learn more on how to become a clinical research professional.
Discover more from Clinical Research Training | Certified Clinical Research Professionals Course
What is the Benefit of a Master's in Clinical Research
Advance your clinical research & administration skills with our masters in clinical research course online. To know more about the online course visit our website now!
A Master's in Clinical Research opens up a wide range of job options. There's a huge market for clinical professionals with experience and expertise in the developing, conducting, and monitoring processes in clinical trials to help transform scientific discoveries and research into possible and usable treatments.
It's a dynamic, high demand profession that fits all lovers of science. Getting a Master's in Clinical Research gives you the skills to develop, conduct, coordinate and monitor clinical studies. It also helps you comply with the protocols of regulatory agencies.
A Master's in Clinical Research can take you to more financially rewarding clinical research career choices and opportunities. It can also set you up to work at a variety of companies, like public and private medical institutions and companies, contract research organizations, hospital systems. Once you are in the field, there is an enormous amount of position choices. Additionally, you can expect a steady promotion to industrial, research, managerial, supervisory or specialist positions.
The minimum requirements you need for your Master's in Clinical Research are either a bachelor's degree in medical sciences, an advanced education in health professions with work experience, or a standardized test score like GREs.
Clinical research masters online will include a combination of the following;
Regulatory and compliance issues
Biostatistics and computing
Epidemiology
Medical informatics
Health services research
Supervised clinical research
Patient-oriented research
Experiential learning in clinical research
Concentration in investigation
Conducting clinical research studies
Health care
Biologics
Health sciences policies
And others
A Master's in Clinical research is a 30 - 33 credit program, depending on the course you want. It is comprised of required courses, program electives, course works, cognate electives, thesis or project, and supervised research. There's a wide range of specialized clinical research masters to go for: clinical research professional, clinical research administration, clinical research coordinator, and others. As an area of medical sciences that is growing tremendously. Demand is so high that the median salary is $82,090 and varies depending on the geographical location and work experience. You can check positions and salaries near you here.
To learn more about the courses offered and how the Master's in Clinical Research program is run, check out this clinical research master's degree program.
Take courses from CCRPS and learn more on how to become a clinical research professional.
Discover more from Clinical Research Training | Certified Clinical Research Professionals Course.
Clinical Research Training For Employers
Clinical Research Training For Employers
No employer in any field or industry employs a staff without a prior knowledge, ability or competence in carrying out their supposed duty and fulfilling their job description. It is the same with the clinical research industry as well. Examples of employers in this industry are pharmaceutical companies, biotech companies, clinical research organizations, government agencies etc. These companies and organizations all require a minimum level of training that individuals must possess before they can be considered employable. There are minimum educational requirements, certification and certain other skills that employers consider before employing a clinical research professional.
However, because of the nature of medical science, the growth and evolution of science and the advancement of technology, here is a constant need for clinical research professionals. This has increased the importance of clinical research training for employers.
Employers who want to increase the level of professionalism and competence among their staffs often enroll them for online and offline training programs. There are different variations of these programs, depending on the training platform. Some of these variations offered by different platforms include:
Classroom training: this program reduces the amount of time spent out of office or work by the staffs. In this program, the trainers come down to the company's facility to train the staffs. This reduces the amount of time the staffs will spend traveling, as well as the travel expenses. This increases the staffs competence and improves their job performance while at work.
Online training: this is a convenient and self-paced electronic based training program that makes use of different adult learning techniques that enables the students (organization's staffs) to fully understand what has been taught. This makes it easy for them to apply what they are learning on the job. This program makes use of different online platforms that ensures interaction between the lecturers and the students. For comprehensive online training, consider courses like the Clinical Research Coordinator, Pharmacovigilance Certification, and ICH-GCP from CCRPS.
Custom program: this program allows the team of trainers to work with the organization's staffs in order to develop online and offline training programs that are tailored to meet the needs and specifications of the organization and its staffs.
Boot camp and onboarding program: programs like these are most usually for clinical research associates and clinical research coordinators. The onboarding program is ideal for new CRAs. It is a unique intensive training program that can be customized to meet the company's needs. These programs allows the new CRAs and CRCs to make immediate impact and saves the organization more time and money. Specialized training for CRAs and CRCs can be found through the CRA Training and Clinical Trials Assistant Training at CCRPS.
Take courses from CCRPS and learn more about how to become a clinical research professional. Advanced certifications such as the Advanced Clinical Research Project Manager Certification and Advanced Principal Investigator Physician Certification can significantly enhance the expertise and capabilities of your team. Additionally, for those in more specific roles, the Medical Monitor Certification is also available.
Discover more from Clinical Research Training | Certified Clinical Research Professionals Course
Clinical Research Associate Training and Placement
The role of the clinical research associate is very important in clinical trials to ensure that medical devices, new treatments and new drugs are approved for patients' use.
This field is taken as a certificate program course in many schools. You may also discover the availability of associate degree programs depending on the school. These programs can be completed in two years and can be offered through both the online and the hybrid formats. Hybrid formats combine both online and on-campus courses together.
If you opt for an online program, different platforms like emails and discussion boards are used to ensure and promote interaction between the students as well as with the lecturers. Online learning platforms are used to upload the syllabus, course materials, lectures and assignments. Some online programs include field work as part of their requirements, in order to gain first hand experience working with clinical trials and patients. Depending on the school, they may have a list of approved clinical research institutes and other facilities. Otherwise, you will have to find a facility for yourself and get the school's approval.
These certificate programs are generally designed for professionals that are already in the medical fields like medical assistants, nurses etc, and are interested in moving to the field of clinical research. They may therefore ask for a copy of your CV or resumé or they may ask for a letter from your employers to verify that you have the needed medical experience. Some programs may require just an undergraduate degree in a medical science or life science related field.
Clinical research associates are trained to assist clinical researchers and investigators in the coordination, administration and management of clinical trials. During this training, different courses will be taught revolving around subjects like safety procedures, subject recruitment, regulatory requirements, drug development, accountability, trial management, medical terminology etc.
The importance of the role of the clinical research associate means that companies that conduct clinical trials are usually very selective, the need to comply with strict regulations often inform their decision when making a choice of their clinical research associate. It is therefore very difficult to get a job as a clinical research associate without previous experience of clinical trials. Many companies require around at least two years experience in clinical monitoring as a clinical project assistant or clinical trial administrator before considering applicants for this important role.
In applying for the post of a clinical research associate, ensure that you read the job description and indicate or highlights the relevant experience on your curriculum vitae. Your cover letter should be specific to the company you're applying to. Do not use a one-for-all cover letter. Personalize your cover letter to each company and highlight the skills that fit the specific requirements of the role. Not all companies advertise their vacancies, so you can try to find out about other unadvertised vacancies.
Take courses from CCRPS and learn more on how to become a clinical research professional. Explore courses like Clinical Research Coordinator, Pharmacovigilance Certification, CRA, ICH-GCP, Advanced Clinical Research Project Manager Certification, and Advanced Principal Investigator Physician Certification.
Discover more from Clinical Research Training | Certified Clinical Research Professionals Course
Contact Us
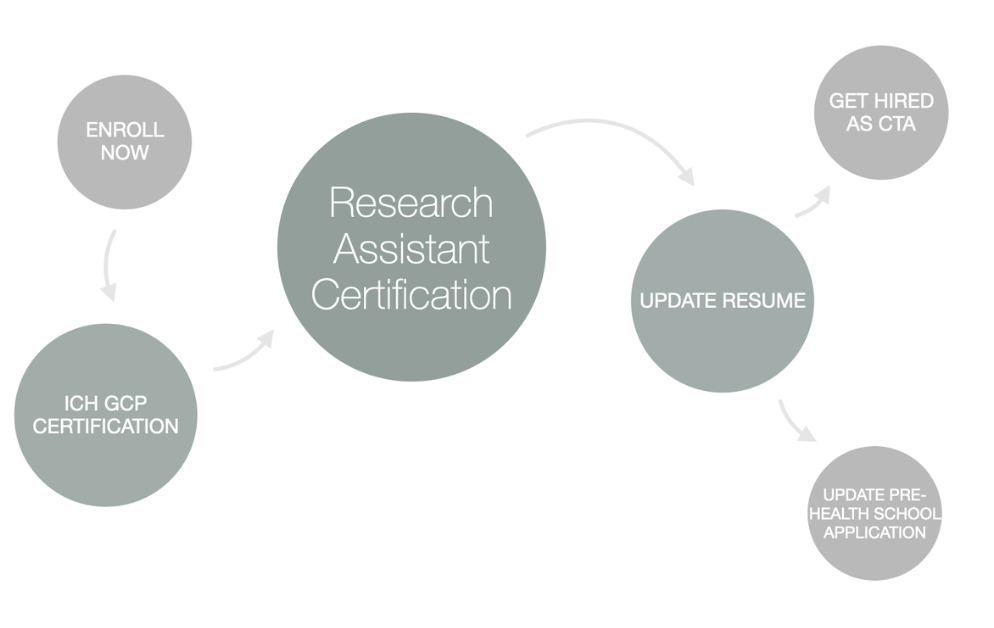
What You Need to Know About Being a CTA (Clinical Trial/Research Assistant)
Get the right clinical research assistant job from CCRPS, Here you can get the all information about clinical research assistant job post. To know more visit us now!
The work of a CTA (clinical trial/research assistant) is one of extreme importance to the clinical research institute. CTAs work in a very busy department. To succeed, they needs to have a keen eye for details and an open mind to learn. They need to be able to ask the right question and find the right solutions.
DUTIES
The CTA helps in the collection and organization of data that is procured from studies. A typical day of a CTA is spent observing and communicating with the volunteers who are recruited to handle these kinds of studies. They are then responsible for analyzing and interpreting the statistics, which helps researchers realize their conclusions and results.
One of the critical tasks of a CTA is performing the different safety and quality checks within their unit. These checks are routinely carried out daily, weekly, or monthly. For example, standard equipment such as freezers and fridges are checked at least twice daily. This is important because they are used for storing samples and medications that needs to be kept in controlled temperature. Even a slight deviation from the controlled temperature can impact the validity of the result and the research.
Additionally, emergency equipment is checked on a daily basis. This ensures the safety of all the staffs and volunteers within the clinical research institute.
Another part of a CTA's job is to assist members of the team and deal with queries from members of the public. A CTA has to work in an administrative capacity and help with research paperwork. There are others who may have to perform some basic medical responsibilities like administering the medication for trials and even drawing blood.
In the midst of these many duties, it is very important that the CTA is good at multitasking. A good communication skill (both written and verbal) is also very important in this line of work.
EDUCATION
Most of the jobs in this area need a person to have a bachelor’s degree within the life sciences. However, there are some employers who require an advanced graduate degree, like a BMSc.
It is important for the clinical research assistants have a strong educational background in the administration of clinical trials. This means that you may have the kind of skills that help in organization. Having an interest in statistics and math can be an added advantage.
If you don’t have a degree in the science field and don’t want to go back to school, finding training and real experiences in clinical trials is going to help you hand a CTA position. At CCRPS, we offer effective courses and experiences you need to get your foot in the door. We have a CTA course and a free ICH GCP certification course, which teaches important skills that will help you succeed in clinical research. If you are looking to advance further, consider our Advanced Clinical Research Project Manager Certification or the Advanced Principal Investigator Physician Certification. For those interested in the crucial aspects of drug safety, our Pharmacovigilance Certification might be the right fit. Explore these to gain deeper insights and practical knowledge.
Take courses from CCRPS and learn more on how to become a clinical research professional.
Discover more from Clinical Research Training | Certified Clinical Research Professionals Course