The Impact of Regulatory Changes on Clinical Trials 2025 Analysis
Starting in 2025, regulatory change is not a “compliance update.” It is a budget and timeline weapon. Sponsors are getting hit with silent delays: submissions bouncing between portals, vendors misreading regional expectations, decentralized workflows creating new documentation gaps, and safety reporting teams drowning in volume and audit pressure. This analysis breaks down what changed, how it impacts startup, conduct, and inspection readiness, and what to do if you want faster approvals and fewer findings. If you work in trials, these shifts also reshape hiring, salaries, and the skills that get promoted.
1. 2025 Regulatory Landscape: What Changed and Why It’s Different
Clinical trials in 2025 are being shaped by one ruthless theme: regulators want demonstrable control, not just good intentions. That means your Quality Management System has to be real, your oversight has to be traceable, and your data integrity has to hold up when someone goes looking for weaknesses.
The first big change is the rise of systems thinking in compliance. Regulators are less impressed by “we trained everyone” and more focused on whether your workflows actually prevent problems. That is why quality roles keep growing and why the fastest way to understand the new expectations is to study how QA operates, not how marketing describes compliance. If you want that perspective, start with the QA specialist career roadmap and link it to GCP fundamentals using the GCP certified professional salary trends report.
Second, global trials have become multi-jurisdiction puzzles. You are not dealing with one regulator. You are dealing with different portals, different documentation norms, different privacy expectations, and different inspection cultures. If your regulatory team is not strong, the study will drift before the first patient is even screened. Build clarity by reviewing the regulatory affairs specialist roadmap, the clinical regulatory specialist pathway, and the execution focused regulatory affairs associate guide.
Third, regulators are not backing down on risk-based oversight. That impacts monitoring, data cleaning, and what you must prove you reviewed and escalated. Sponsors that still treat oversight like a box-check are the ones paying for late-stage remediation. Tie this to practical tool stacks and monitoring models using the top 50 remote clinical trial monitoring tools and data foundations from the clinical data manager roadmap.
Finally, safety expectations feel heavier in 2025 because the operational load is heavier. More trials, more endpoints, more reporting pressure, more scrutiny on narrative quality. If your safety function is weak, it turns into compliance risk fast. Use the drug safety specialist career guide and the pharmacovigilance manager pathway to understand what “good” looks like in modern PV operations.
2. Study Startup Under New Rules: Feasibility, Protocols, and Submissions
In 2025, study start-up fails in quieter ways than before. You are not always “rejected.” You get stuck in clarification loops. You lose weeks to portal friction, document mismatch, and incomplete evidence of oversight.
The biggest start-up trap is feasibility that is built on hope. When regulators and ethics bodies see unrealistic recruitment assumptions, protocols often get amended, and amendments are expensive. You need feasibility that is anchored to real site capacity and real patient flow, not optimistic spreadsheets. Use the sponsor-grade recruitment ecosystem view from the patient recruitment companies and tech solutions mega list and validate site strength through the top academic medical centers with active clinical trials list.
Protocol design is also being punished for complexity. Complexity is not a badge. It is deviation fuel. The more complicated your workflow is, the more you need documented controls, and the more your oversight burden increases. This is where data and monitoring need to be aligned from day one. Tie start-up decisions to data reality using the clinical data coordinator pathway and long-term data governance through the clinical data manager roadmap.
Regulatory submissions now demand packaging discipline. Sponsors who lack strong regulatory operations often treat submissions like a one-time upload instead of a controlled narrative. That is why regulatory career paths matter. A sponsor that understands the real work of regulatory teams can avoid 80 percent of avoidable delays. Ground your expectations using the regulatory affairs specialist roadmap, the clinical regulatory specialist pathway, and the execution-focused regulatory affairs associate guide.
One more start-up reality in 2025: oversight must be visible. If you outsource, you still own outcomes. Regulators expect sponsors to prove that CROs and vendors are controlled. If you do not have a governance rhythm, your program becomes a blame factory. For sponsor-side accountability and escalation design, study how leadership roles operate in practice through the clinical research administrator pathway and budget-driven oversight signals like the clinical research project manager salary trends guide.
3. During the Trial: Monitoring, Data, Safety, and Documentation Under Tighter Expectations
Once the trial is live, 2025 regulatory change hits where it hurts: day-to-day execution. That is where most teams quietly lose control.
Monitoring is no longer about proving you visited a site. It is about proving you saw risk early, acted, and documented the decision chain. If you cannot reconstruct why you ignored a signal, you are exposed. Sponsors are shifting toward centralized and hybrid oversight, which raises documentation expectations and tools discipline. Build a modern monitoring view using the top remote monitoring tools and platforms guide and connect it to the skill foundation of CRAs via the clinical research associate salary report.
Data is where small sloppiness becomes regulatory risk. Late entry behavior, inconsistent source, uncontrolled spreadsheets, and fuzzy reconciliation plans are not “site problems.” They are governance failures. If your data strategy is weak, your lock timeline becomes fantasy, and your team bleeds in rework. Strengthen your data understanding through the lead clinical data analyst career guide and compare platform realities with the top 100 clinical data management and EDC platforms directory.
Safety reporting is also more painful because workload and expectations have both increased. In 2025, weak PV operations create a slow-motion disaster: backlogs, inconsistent narratives, delayed escalations, and poor signal documentation. If you want to understand why PV capability is becoming a sponsor-level advantage, study the operational ladder from the pharmacovigilance associate career roadmap to the drug safety specialist guide and leadership competencies in the PV manager roadmap.
Documentation is the silent killer. Many teams think documentation is a clerical afterthought. In 2025, documentation is evidence of control. If documentation is late, inconsistent, or missing, regulators interpret that as weak oversight even if the trial “felt fine.” This is why QA keeps growing and why teams with mature QA partnerships avoid expensive remediation. Build that mentality through the QA specialist roadmap and reinforce discipline with exam-level execution habits from proven test taking strategies.
4. Inspection Readiness and Quality Systems: How to Avoid Findings in 2025
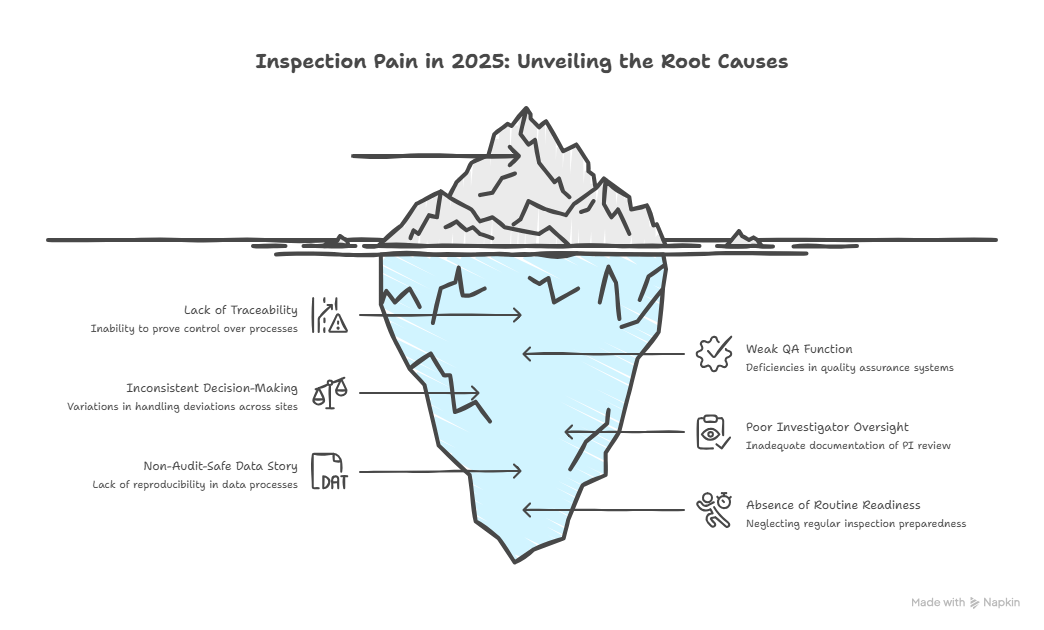
Most inspection pain in 2025 is preventable. It comes from one pattern: you cannot prove you were in control. You may have done the work, but you cannot show the evidence chain.
Start with a brutal inspection readiness mindset: regulators do not audit your intentions, they audit your traceability. That is why the Trial Master File, oversight logs, decision records, vendor KPIs, and deviation trending matter so much more than slide decks. If your QA function is weak, you will discover it during the worst possible week. Use the QA specialist roadmap as a blueprint for what regulators expect from quality systems, then anchor GCP basics via the GCP certified professional insights.
The second inspection readiness rule is consistency. In 2025, inconsistent decision-making reads like weak governance. If two sites handle deviations differently, or two safety narratives look like different products, auditors suspect systemic weakness. Tighten consistency by standardizing workflows, then auditing the workflow itself, not just the outputs. This is where sponsor PM and administration roles become critical. Learn how internal leadership tracks operate using the clinical research administrator pathway and the execution pressures visible in the clinical research project manager salary guide.
Third, your investigator oversight must be clean. In 2025, delegation and PI oversight issues keep showing up because trial complexity has increased. If PI review is not documented, you are exposed. If sub-investigator roles are unclear, you are exposed. Refresh your governance understanding through the principal investigator roadmap and the sub-investigator pathway.
Fourth, your data story must be audit-safe. It is not enough to have clean datasets. Regulators want to know how you got there. Your edit checks, query decisions, reconciliation plans, and lock governance must be reproducible. Build a practical CDM mindset using the clinical data manager roadmap, and push deeper into leadership execution through the lead clinical data analyst guide.
Finally, treat readiness like a routine, not a panic. A simple way to do that is to run a monthly “inspection pack” review and train teams using test-based discipline. If your organization struggles with operational discipline, use frameworks like proven test taking strategies and environment control from creating the perfect study environment to enforce consistency.
5. Career and Hiring Impact: Which Roles and Skills Spike With Regulatory Change
Regulatory change always reshapes who gets hired. In 2025, the winners are the people who reduce risk without slowing trials.
Regulatory and clinical regulatory roles are rising because submissions are more complex and global execution is harder. If you want to enter or level up, build your path using the regulatory affairs associate guide, then deepen into the clinical regulatory specialist pathway and the broader regulatory affairs specialist roadmap.
Quality roles are climbing because modern trials need systems proof. QA is not “policing.” It is a competitive advantage when you can prevent findings while still moving fast. That is why QA keeps showing up on compensation lists and leadership tracks. Start with the QA specialist roadmap and benchmark where the pay gravity is using the clinical research salary report 2025.
Data roles are expanding because regulators care about integrity and traceability. Data is not a back-office function anymore. It is a compliance function. The most valuable data professionals are the ones who can build audit-safe pipelines and prevent query chaos. Use the clinical data coordinator pathway, the clinical data manager roadmap, and the tooling landscape in the top 100 EDC platforms directory.
Safety and PV roles remain strong because safety workload is growing and scrutiny is high. If you want a path that stays relevant, PV offers clear ladders and strong specialization. Start with the PV associate roadmap, expand into execution via the drug safety specialist guide, and then target leadership through the PV manager pathway.
Project management and governance roles are also rising because regulatory change increases cross-functional complexity. Sponsors need leaders who can enforce clarity across CROs, sites, data, safety, and regulatory. That is why governance-heavy roles remain lucrative and why understanding PM scope is practical, not optional. Use the clinical research project manager salary trends guide and the broader pay lens from the top 10 highest paying clinical research jobs.
6. FAQs: Regulatory Changes and Clinical Trials in 2025
-
The shift from “documented compliance” to provable control. Regulators want to see that risks were identified, monitored, acted on, and documented with traceable oversight. This elevates QA, RBQM, and documentation discipline. Build a practical lens through the QA specialist roadmap and the GCP foundation in the GCP certified professional insights.
-
They increase the cost of sloppiness. Weak submissions, incomplete packages, unrealistic feasibility, and unclear oversight plans create clarification loops and rework. The fix is a region-specific submission readiness checklist plus feasibility grounded in real site capacity. Strengthen regulatory execution using the regulatory affairs associate guide and expand into strategy with the regulatory affairs specialist roadmap.
-
Yes, because they create more handoffs, more vendors, and more documentation points that must be traceable. The failure pattern is remote activity without clear evidence of oversight. Use modern oversight and monitoring models from the remote monitoring tools guide and ensure your data workflows are audit-safe through the clinical data manager roadmap.
-
Because regulators interpret missing or inconsistent evidence as weak governance. Even if the trial ran well, you must prove the decision chain. Sponsors that treat the TMF, oversight logs, and deviation trends as living systems are the ones that pass cleanly. This is why QA discipline matters and why the QA specialist roadmap is one of the highest leverage reads in 2025.
-
They increase expectations around timeliness, narrative consistency, and signal governance. PV failures create high-visibility compliance risk. Strong PV operations are becoming a strategic differentiator. Learn the operational ladder through the drug safety specialist guide and leadership readiness using the PV manager pathway.
-
Regulatory operations, clinical regulatory, QA, CDM, PV, and governance-heavy project management roles. These functions reduce risk while keeping trials moving. Benchmark compensation and demand using the clinical research salary report 2025 and explore high-pay trajectories with the top 10 highest paying clinical research jobs.
-
Build a simple, repeatable operating system: top risk register, RBQM triggers, monthly vendor KPI pack, monthly TMF completeness checks, and documented escalation outcomes. Then train teams using competency-based checks, not passive training logs. For discipline frameworks, use proven test taking strategies and execution consistency principles from creating the perfect study environment.