Sub-Investigator Career Pathway: Clear Steps and Responsibilities
Becoming a Sub-Investigator (Sub-I) is one of the fastest ways for clinicians and researchers to step into leadership inside clinical trials without immediately taking full Principal Investigator responsibility. A strong Sub-I can turn an average site into a high-performing one that sponsors want to keep using. In this guide, you will see exactly what Sub-Is do, how they differ from coordinators and PIs, what skills hiring managers look for, and how to map your next three to five career moves using real-world tactics, directories, and training resources from CCRPS.
1. What Is a Sub-Investigator in Clinical Trials?
A Sub-Investigator is a physician or qualified clinician who supports the Principal Investigator (PI) in conducting protocol-specific medical activities, documenting safety, and ensuring participant care is consistent with regulatory expectations. At high-performing sites listed in the top 100 clinical trial sites directory, Sub-Is are treated as strategic partners, not “backup doctors.” They co-sign orders, review eligibility, and handle protocol deviations, which directly influences how sponsors rank the site alongside those in the best clinical trial sponsors in the US.
Unlike a Clinical Research Coordinator, who manages day-to-day logistics, or a Clinical Trial Assistant, whose duties are more administrative, the Sub-I is responsible for medically significant decisions. This includes reviewing adverse events, confirming inclusion and exclusion criteria, and aligning with pharmacovigilance standards similar to those applied in roles covered in the top 100 pharma and biotech PV employers list. Many Sub-Is later transition into PI or Medical Monitor roles, especially if they leverage resources such as the principal investigator terminology guide to sharpen their vocabulary and documentation clarity.
Sponsors and CROs evaluate sites on speed, quality, and reliability. Sub-Is influence all three. When Sub-Is understand monitoring expectations described in the CRA monitoring terminology guide and data expectations from the clinical data manager terms reference, they anticipate queries, prevent delays, and make monitoring visits smoother. Strong Sub-Is also support decentralized and hybrid trials, aligning with trends discussed in CCRPS articles on decentralized clinical trials and remote monitoring tools.
Finally, the Sub-I is often the bridge between protocol and real clinical practice. They negotiate feasibility when complex protocols arrive from sponsors or CROs listed in the worldwide CRO directory. By understanding regulatory expectations explained in the regulatory affairs specialist roadmap and regulatory terms glossary, Sub-Is help PIs design workflows that keep the study both feasible and compliant.
2. Core Skills, Competencies, and GCP Knowledge Map
The strongest Sub-Is combine clinical judgment, regulatory literacy, and operational awareness. Many start by mastering terminology from resources like the CRA term guide, PV specialist glossary, and the 100-clinical-acronym reference. This vocabulary directly improves their ability to interpret protocols and respond to complex queries from sponsors, CROs, and data management teams.
Clinically, Sub-Is must be able to balance protocol rigidity with patient safety. That requires a deep understanding of disease states, concomitant medications, and standard-of-care treatments. When they read about advanced trial designs, such as those using AI for trial optimization or blockchain-backed data integrity, they can translate these abstract concepts into practical decisions at the bedside. Sub-Is who understand regulatory and quality expectations often work well with QA professionals who follow paths described in the quality assurance specialist roadmap and clinical quality auditor guide.
Operationally, Sub-Is need strong communication and documentation discipline. They must collaborate with coordinators who follow paths outlined in the clinical research assistant career roadmap, CTAs described in the clinical trial assistant guide, and clinical administrators and compliance leads found in the clinical research administrator pathway and clinical compliance officer guide. This cross-functional literacy is one of the fastest ways to become indispensable at any research site or academic medical center.
3. Step-by-Step Career Pathway to Sub-Investigator
There is no single route into a Sub-I role, but the most reliable pathways share common stages. Many physicians start their journey by getting exposure through academic centers listed in the top academic medical centers directory. Residents or fellows working in these centers often assist with data collection, consent discussions, or protocol feasibility, which gives them a first look at how PIs and Sub-Is operate during high-stakes trials.
The next step is usually formal training and role clarity. Clinicians often complete advanced GCP courses and specialized programs such as CCRPS certifications, while simultaneously studying terminology guides for trial project managers and clinical data managers. Some clinicians first accept roles similar to a Clinical Medical Advisor, using roadmaps like the clinical medical advisor career path to practice protocol interpretation, KOL communication, and safety assessment before directly stepping into Sub-I responsibilities.
To secure a first official Sub-I position, leverage directories and networking. Go through regional lists like the top hospitals and health systems running trials, Asia-Pacific clinical trial sites, or European clinical trial sites. Look for centers that already run complex phase II or III programs or emerging decentralized trials. These usually have enough study volume to justify multiple Sub-Is and may welcome motivated clinicians who show they understand PV concepts from guides like the essential PV terms list.
Once in role, you must actively position yourself for upward mobility. This includes volunteering to lead particularly difficult protocols, supporting quality audits alongside QA and compliance teams, and collaborating with CRO partners identified in the global CRO directory. Over time, these experiences create a straightforward path to roles such as PI, Medical Monitor, or even Regulatory Affairs leader, using career guides like the regulatory affairs associate roadmap and clinical regulatory specialist pathway as planning tools.
What’s Your Biggest Challenge in Becoming a Sub-Investigator?
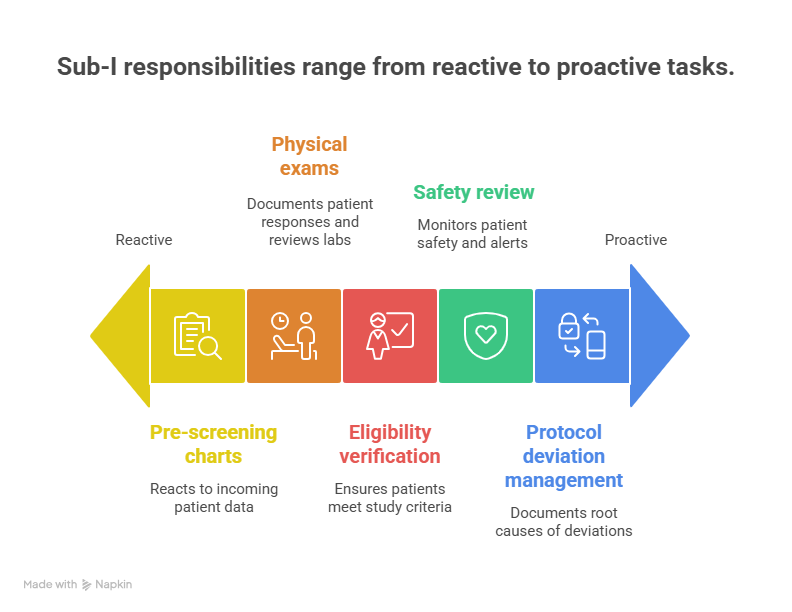
4. Daily Responsibilities, Workflows, and Site Metrics
On a typical day, a Sub-I may move from pre-screening charts to performing on-protocol physical exams, documenting responses, and reviewing lab results. The most effective Sub-Is use tools similar to those featured in the clinical data management platforms guide and remote monitoring platforms list to quickly check outstanding queries and safety alerts. They coordinate closely with CRAs and project managers who follow best practices from the clinical project manager terminology guide, which keeps timelines intact.
Key responsibilities include eligibility verification, safety review, and protocol deviation management. When deviations occur, Sub-Is must work with quality and compliance colleagues using frameworks from the clinical compliance officer guide and quality auditor pathway to document root causes. They also need to understand how their decisions impact downstream pharmacovigilance workflows in global teams covered in the PV training and certification guide, especially when serious events require expedited reporting.
Sponsors track recruitment rate, data quality, and safety profile for each site. Sub-Is directly influence all three metrics. For recruitment, they coordinate with patient recruitment vendors listed in the patient recruitment companies directory. For data, they review CRFs and queries alongside coordinators trained using guides like the clinical trial assistant roadmap. For safety, they collaborate with PV case processing teams similar to those in the remote PV jobs list. Consistently strong metrics make a Sub-I an obvious candidate for PI or regional leadership roles.
5. Salary, Remote Options, and Long-Term Growth
Sub-Investigator compensation varies widely based on geography, specialty, and whether the role is attached to a large healthcare system or a private site network. In markets with heavy trial activity, such as those covered in the US sponsor directory and the global CRO listing, Sub-Is can negotiate higher per-protocol stipends or even partial FTE support from sponsors. Many PIs split compensation with Sub-Is based on visit volume and responsibilities, particularly when Sub-Is take on complex safety and oversight tasks.
Remote and hybrid opportunities are expanding quickly. With the rise of decentralized and hybrid studies outlined in CCRPS articles on decentralized clinical trials and AI-driven trial transformation, Sub-Is may supervise telehealth visits, oversee home-health providers, and review eSource data without being on site every day. This mirrors trends seen in remote-first roles described in the remote monitoring tools guide and remote PV positions in the PV jobs directory.
Long term, the Sub-I role becomes a launchpad into multiple senior tracks. Experienced Sub-Is can become PIs at academic centers listed in the top academic medical centers directory, move into medical leadership roles at CROs or sponsors, or pivot into specialized areas such as pharmacovigilance, regulatory affairs, or quality. Career routes are mapped across CCRPS guides for clinical medical advisors, regulatory specialists, quality specialists, and clinical compliance officers. Sub-Is who intentionally collect diverse trial experience across indications, phases, and technologies will have the greatest negotiating power.
6. Sub-Investigator FAQs
-
In most interventional drug and device trials, Sub-Investigators are physicians because they must perform protocol-defined medical acts, confirm eligibility, and sign off on safety assessments. However, in some observational studies or minimal-risk protocols, advanced practice providers such as nurse practitioners or physician assistants may act as Sub-Is if local regulations and sponsor policies allow it. To understand how different roles are defined across trials, review CCRPS resources on principal investigator terminology and clinical research associate terms, then cross-check with your institutional policies.
-
Start by aligning with sites already running trials in your specialty, using directories like the top clinical trial sites in Europe, Asia-Pacific site directory, or regional hospital lists such as the top US hospitals running trials. Offer to help with chart pre-screening, consent discussions, or safety reviews. While doing so, complete advanced GCP and focused CCRPS training, plus terminology guides for CRAs and project managers. Within 6–18 months, you can credibly request formal Sub-I delegation on new protocols.
-
Titles vary by institution and sponsor. In many studies, Sub-Investigator, Co-Investigator, and Associate Investigator refer to team members who support the PI with protocol-related tasks and have duties documented on the delegation log. Some institutions reserve “Co-Investigator” for individuals with major scientific input, while “Sub-Investigator” emphasizes clinical execution. Regardless of title, responsibilities are defined in the delegation log and must be compatible with GCP expectations described in CCRPS glossaries such as the 100-acronym reference and regulatory term guide.
-
Early in your Sub-I career, prioritize depth over volume. One or two complex protocols can teach you far more about safety, deviations, and data quality than five superficial ones. Choose studies with strong operational support from coordinators following guides like the clinical research assistant roadmap or clinical trial assistant guide. As you become comfortable with GCP expectations and monitoring visits, you can gradually expand your portfolio, focusing on indications and sponsors that align with your long-term goals, such as PV leadership or regulatory strategy.
-
Yes, decentralized and hybrid designs are opening new remote pathways for Sub-Is. In these models, you may conduct telehealth visits, review eSource data, supervise home-health nurses, and handle virtual safety reviews. Articles such as the CCRPS guide on decentralized trials eliminating traditional sites and the remote monitoring platforms directory explain the technology stack that makes this possible. Remote work still requires robust local backup for emergencies and clear SOPs so that your oversight remains consistent with GCP and local regulations.
-
Promotion to PI usually depends on demonstrated leadership, audit-ready documentation, and sponsor trust. To accelerate this, choose high-visibility protocols with demanding endpoints, then partner closely with CRAs and QA teams who follow frameworks in the quality assurance specialist roadmap and clinical quality auditor guide. Present your site’s performance at investigator meetings and volunteer to host sponsor or regulatory inspections. Over time, combine this experience with educational resources covering PI-specific terminology and responsibilities using the PI term guide, then negotiate PI status when new protocols are awarded to your institution.
-
While you do not need to work full time in pharmacovigilance, a strong understanding of PV concepts is essential because Sub-Is are responsible for accurate AE detection, causality assessment, and timely reporting. Familiarity with PV terms from CCRPS resources like the PV terminology guide and insight into employers listed in the top PV companies directory will help you speak the same language as sponsor safety teams. This capability is one of the clearest signals that you are ready for higher-level medical leadership roles in drug development.