Guide to Becoming a Lead Clinical Research Coordinator: Career Steps and Skills
You can be an excellent Clinical Research Coordinator and still feel stuck. You are doing the visits, chasing labs, fixing queries, calming anxious patients, and cleaning deviations after the fact. Then you watch someone else get promoted to Lead. The difference is rarely “worked harder.” It is leadership behaviors, inspection ready execution, and proof you can run a site like a business. This guide shows the exact steps and skills to move from CRC to Lead Clinical Research Coordinator with a promotion ready plan, real deliverables, and the language that sponsors and managers trust.
1: What a Lead Clinical Research Coordinator Actually Owns (Beyond “More Experience”)
A Lead Clinical Research Coordinator is the person who makes a study run predictably, even when everything is messy. You are not just executing tasks. You are owning outcomes across quality, timelines, enrollment, documentation, and communication. If you want the title, you need to prove you can create stability inside chaos, the same way a strong clinical research administrator career pathway proves operational leadership, or a high performing principal investigator career roadmap proves clinical accountability.
Here is what “Lead” usually means in real life:
Quality owner: You prevent protocol deviations instead of explaining them later. You maintain inspection readiness like a habit, not a scramble. Learn how quality roles think by studying the QA specialist career roadmap.
Study operations driver: You create workflows for source, CRFs, queries, logs, and closeout. You reduce rework, which is where coordinator teams lose time and morale. This aligns closely with a strong clinical data coordinator career path mindset.
Sponsor facing communicator: You can summarize risk, status, and next actions in two minutes, and you write emails that do not create confusion. That communication strength is also what separates strong professionals in regulatory affairs associate career steps.
Trainer and multiplier: You onboard new CRCs, fix gaps without humiliating people, and standardize what “good” looks like. Your value becomes scale, similar to what you see in a pharmacovigilance associate career roadmap where progression comes from owning a system, not a task list.
Risk manager: You anticipate what will break. You track bottlenecks, build buffers, and keep the PI out of trouble. If you want more context on how risk is measured at higher levels, review the clinical medical advisor career path and how leaders communicate safety signals.
The pain points that prove you are ready for Lead
If you recognize these, you are already doing Lead level work, you just have not packaged it:
You are tired of being the “fixer” who cleans errors created by unclear processes. A Lead creates the process and reduces the errors, like the best clinical data manager career roadmap operators do.
You hate last minute audit panic. A Lead maintains inspection readiness daily, similar to quality principles inside the clinical regulatory specialist pathway.
You are already coaching others, but you are not getting credit. A Lead turns coaching into documented onboarding, checklists, and measurable improvement, just like the frameworks taught in creating the perfect study environment.
You feel pulled between patient care and sponsor demands. A Lead creates predictable workflows so patient experience does not suffer, which is a mindset you also see in the sub investigator career pathway.
| Competency Area | What “Lead Level” Looks Like | Proof Artifacts (What You Can Show) | How to Build It (30–60 Days) |
|---|---|---|---|
| Inspection Readiness | Maintains readiness weekly, not “before audit” | Readiness checklist, monthly binder review notes | Run weekly mini-audit, log findings, fix patterns |
| Delegation | Assigns tasks with clear deadlines and standards | Delegation matrix, task tracker snapshots | Create RACI by study and review in huddles |
| Protocol Mastery | Knows protocol risk points and visit critical path | Visit flow map, risk notes per visit window | Create one-page “visit truth sheet” per protocol |
| Deviation Prevention | Prevents recurring deviations through process | Deviation root cause log, prevention SOP | Track top 3 deviations, write fixes, train team |
| Query Management | Predictable query turnaround and zero “surprise” backlog | Query KPI chart, weekly query review notes | Set daily query blocks + escalation rules |
| Source Quality | Source is complete, contemporaneous, and consistent | Source templates, completeness checklist | Standardize templates for common assessments |
| eReg / eISF | Folder structure, naming, and QC are standardized | Naming SOP, QC log, corrected examples | Implement naming rules + weekly QC sampling |
| Enrollment Strategy | Owns screening funnel and identifies drop-off points | Screen fail tracker, funnel dashboard | Track reasons weekly, tighten pre-screen criteria |
| Patient Experience | Visits run on time, patient confusion minimized | Visit script, patient prep checklist | Create pre-visit call script + post-visit summary |
| IP Accountability | Zero temperature excursions without documentation | IP logs, excursion CAPA, training record | Build daily IP check routine + backup plan |
| Lab / Sample Chain | Specimens shipped correctly, no repeat draws | Sample checklist, shipping training cheat sheet | Create “draw-to-ship” one-pager for staff |
| Vendor Coordination | Vendors are managed proactively, not reactively | Vendor contact map, issue tracker | Set escalation SLAs and recurring vendor sync |
| Monitor Relationships | CRAs trust your answers and your documentation | Monitor follow-up log, resolved action list | Send pre-IMV readiness update + action closure |
| Meeting Leadership | Runs huddles that end with owners and deadlines | Agenda template, action item list | Weekly 20-min huddle with strict format |
| Training | Onboards new CRCs with measurable competence | Onboarding plan, skills checklist, signoffs | Create 14-day onboarding track + shadow plan |
| Documentation Writing | Writes notes that reduce questions and risk | Redlined examples, “gold standard” notes | Rewrite 10 notes, compare to query outcomes |
| Informed Consent Flow | Consent errors near zero, process consistent | Consent checklist, retraining record | Implement consent QC before every randomization |
| Data Integrity | Prevents transcription errors through workflow | Double-check SOP, audit trail review steps | Define critical fields and 2-step verification |
| Recruitment Partnerships | Maintains referral sources and outreach cadence | Referral tracker, outreach scripts | Monthly outreach schedule + tracking sheet |
| Budget Awareness | Understands visit cost drivers and avoids waste | Time-on-task notes, “waste” reduction list | Track rework hours, propose 3 fixes to manager |
| Closeout Readiness | Closeout is organized, minimal last-minute rework | Closeout checklist, final reconciliation log | Run monthly “closeout as you go” checks |
| CAPA Mindset | Uses root cause and prevention, not blame | CAPA templates, completed CAPA examples | Write CAPA for recurring issue and train team |
| Stakeholder Summaries | Can summarize status in 90 seconds | Weekly status email template, dashboard | Create 5-metric weekly status report per study |
| Workload Planning | Anticipates peak weeks and rebalances staff | Capacity planner, coverage calendar | Build visit forecast + staffing coverage plan |
| Conflict Handling | Resolves issues calmly with evidence and options | Issue logs, resolution notes | Use “problem / impact / options” framework |
| Cross-Study Standardization | Creates common systems across protocols | Standard folders, templates, SOP snippets | Standardize naming, logs, and checklists |
| Regulatory Support | Submissions and essential docs never “missing” | Reg doc tracker, submission checklist | Monthly doc reconciliation + expiry alerts |
| Mentorship | Develops junior CRCs into independent operators | Mentorship notes, skill progress snapshots | Weekly 1:1 coaching with specific skill goals |
| Promotion Packet | Presents a business case for Lead title | One-page impact summary + proof folder | Compile artifacts into a clear promotion deck |
If you want the promotion, your job is to convert “I do a lot” into “I produce outcomes.” That means you need evidence, which we will build.
2: The Step by Step Career Path from CRC to Lead CRC (With a Promotion Ready Plan)
Most coordinators wait for permission. Leads build proof first, then ask for the title. Your best strategy is to run a 60 to 90 day plan that creates artifacts your manager can trust, similar to how candidates prove readiness in a structured clinical research assistant career roadmap or a progression focused clinical trial assistant career guide.
Step 1: Clarify what “Lead” means at your site
Some sites define Lead as “senior CRC on the hardest protocol.” Others define it as “team lead and trainer.” Ask for the definition in writing, then map your proof to that definition. When you speak in role definitions, you sound like leadership. This is how professionals progress in structured paths such as regulatory affairs specialist career roadmap or in the systems driven work of a clinical data manager roadmap.
What to ask your manager:
What outcomes would make you say I am already functioning as a Lead?
Which studies need a Lead level coordinator right now?
Which gaps create the most rework for you, the PI, and monitors?
Step 2: Pick one “flagship” study where you can lead visibly
Trying to lead everywhere at once becomes invisible. Choose one protocol where you can run tight execution and produce measurable change. This mirrors how specialized operators build credibility in pharmacovigilance manager career steps or how analytics leaders earn trust in a lead clinical data analyst career guide.
Your flagship study should have at least one of these problems:
High query volume or slow query turnaround
Frequent deviations around visit windows, labs, or dosing
Enrollment drop off after screening
Messy documentation that triggers CRA escalations
If you can stabilize a messy study, you prove Lead readiness fast.
Step 3: Build a weekly operating system
Leads do not “remember everything.” They design systems so nothing depends on memory. Start with a weekly cadence:
Monday: visit forecast, staffing coverage, open action items
Daily: query block, eReg QC sampling, patient follow ups
Thursday: monitor readiness, unresolved issues list
Friday: status summary, risks, next week priorities
This kind of cadence is how you transition from task worker to operator, a shift you also see in leadership tracks like clinical research administrator pathway and compliance heavy functions like clinical regulatory specialist steps.
Step 4: Create a “proof folder” from day one
You do not need a long speech. You need evidence. Build a folder with:
Before and after metrics: query count, turnaround time, deviations
Templates you created: visit checklists, source templates, huddle agenda
Examples of sponsor communication: weekly status emails
Training artifacts: onboarding checklist, skills sign offs
If you want more structure, borrow how certification learners build organized systems in proven test taking strategies and apply the same discipline to your workplace proof.
Step 5: Ask for the title when you are already functioning as Lead
Your promotion conversation should sound like this:
“I have been acting as Lead on X study for 8 weeks. Here are the outcomes, here is the system, and here is how it reduces risk for the PI and sponsor. I want the Lead CRC title aligned to the responsibilities I am already delivering.”
This is the same framing used in role transitions such as drug safety specialist career advancement and in more senior clinical paths like the clinical medical advisor career path. It works because it is evidence based, not emotional.
3: The Core Skills That Separate a Lead CRC from a Strong CRC
The title is not granted for being busy. It is granted for controlling risk, quality, and throughput. These are the skills that create that control.
Skill 1: Inspection ready documentation and data integrity
Lead CRC documentation is consistent. It reduces questions. It prevents contradictions between source and eCRF. That integrity matters because it protects patients and prevents sponsor distrust, which becomes career limiting fast. If you want to understand how documentation becomes a career differentiator, study the systems thinking in clinical data management platforms guide and apply those principles to source creation and QC.
Practical moves:
Standardize source templates for common assessments.
Define “critical fields” that always get a second check.
Build a weekly QC routine that samples charts before monitors find issues.
Skill 2: Protocol risk mapping and deviation prevention
Leads do not treat deviations like accidents. They treat them like predictable outcomes of weak systems. Your job is to map the protocol risk points, then design safeguards. This is identical to how high responsibility clinical roles operate in the sub investigator pathway and how compliance oriented teams think in the QA specialist roadmap.
Practical moves:
Create a one page visit flow with time windows and critical procedures.
Track your top 3 deviation types and write root cause plus prevention.
Train the team on prevention, not on excuses.
Skill 3: Sponsor communication that builds trust
Sponsors want clarity and predictability. Monitors want fast closure and clean documentation. If your communication reduces their stress, you become the coordinator they request. That is how you become Lead. Strong communication is also the difference maker in sponsor facing careers such as medical science liaison career roadmap and advanced paths like medical science liaison and medical monitor salary insights where credibility is everything.
Practical moves:
Use a weekly status template: enrollment, deviations, queries, risks, next actions.
When you escalate, always include options and a recommendation.
Close action items with evidence, not just “done.”
Skill 4: Leadership without authority
Many coordinators fail here. They wait for the title before acting like a Lead. Leads do the opposite. They coach, standardize, and hold standards calmly. Leadership without authority is also what allows professionals to grow across roles such as regulatory affairs specialist roadmap and senior clinical tracks like the principal investigator roadmap.
Practical moves:
Run 20 minute huddles with agenda and action items.
Create a skills checklist for new CRCs and sign off competence.
Give feedback using evidence: “Here is the standard, here is the gap, here is the fix.”
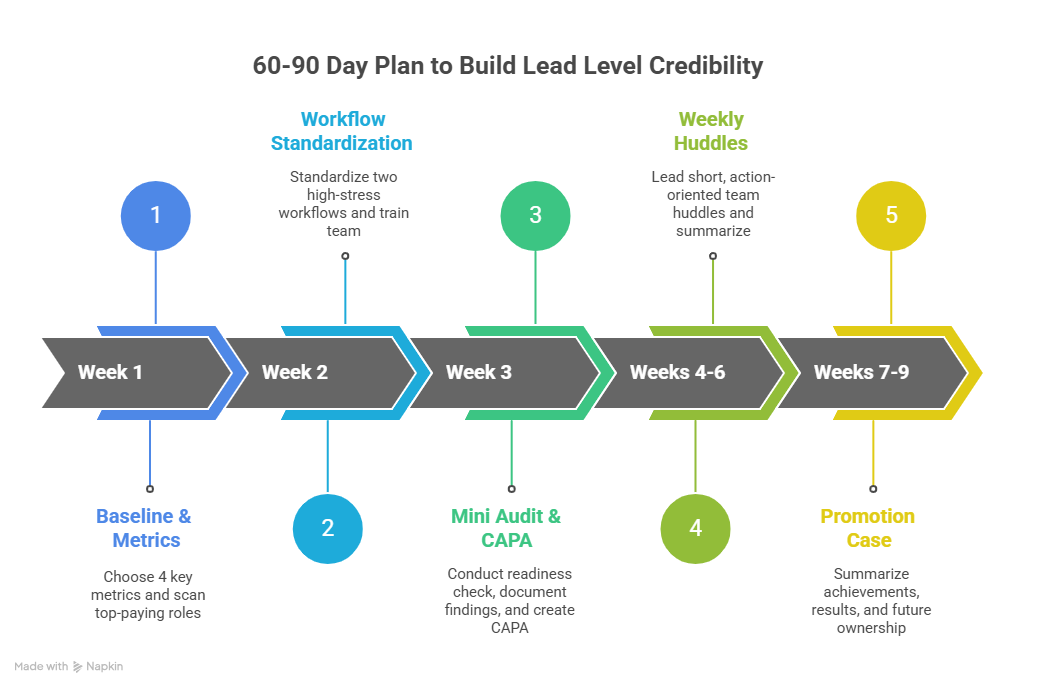
4: How to Build Lead Level Credibility in 60 to 90 Days (What to Do This Week)
You do not become a Lead by learning theory. You become a Lead by shipping systems that reduce risk and rework. Here is a practical execution plan that works in real sites with real chaos, the same way structured progress frameworks help in guides like clinical research salary report and growth focused roadmaps like clinical data manager steps.
Week 1: Set your baseline and pick your metrics
Choose 4 metrics your manager and monitors care about:
Query backlog and average closure time
Deviation count and repeat deviation types
Screening to enrollment conversion
Essential document QC findings per week
If you want to pressure test how pay ties to performance and responsibility, scan the top 10 highest paying roles in 2025 and notice how responsibility scales with risk ownership.
Week 2: Standardize two workflows that remove daily pain
Pick two workflows that create the most stress for everyone:
Consent and screening workflow
Source completion and query handling workflow
Publish a simple checklist and train the team. This is what leadership looks like. It is also how specialized roles build trust, like in clinical regulatory specialist steps and regulatory affairs associate guide.
Week 3: Run your first mini audit and create a CAPA
Do not call it an audit if that word creates fear. Call it a “readiness check.” Review consent files, key logs, and a small sample of source against eCRF. Document findings, root cause, and prevention. That simple CAPA mindset is what separates “busy” from “ready,” and it mirrors how quality teams operate in the QA specialist career roadmap.
Week 4 to 6: Lead a weekly huddle and own action closure
Your huddle is where you become a Lead in front of your team. Keep it short. End with action items, owners, and deadlines. Send a summary. This builds trust the same way consistent reporting builds credibility in sponsor facing paths like medical science liaison roadmap and higher accountability roles like clinical medical advisor pathway.
Week 7 to 9: Package your promotion case
Build a one page summary:
What problem existed
What system you built
What results changed
What risks you reduced
What you will own as Lead going forward
Use clean numbers, not emotional language. Managers promote people who reduce their risk. That is the same logic behind why sponsors pay more for specialists, shown in role focused reports like pharmacovigilance specialist salary growth and project leadership pay in the clinical research project manager salary guide.
5: Interview and Promotion Packet Skills That Make You the Obvious Choice
Many coordinators fail the promotion step because they speak like workers, not operators. Your goal is to present yourself as someone who can run studies with predictable outcomes, like leaders in clinical data management or compliance heavy functions such as regulatory affairs specialist.
The Lead CRC language that wins
Use language that proves control:
“I built a workflow that reduced repeat deviations by X.”
“I created a weekly readiness check and closed findings within 5 business days.”
“I standardized source templates to reduce monitor follow ups and transcription errors.”
“I run a weekly huddle and track action closure, so nothing sits unresolved.”
That is how leaders speak across the industry, whether they are advancing in a drug safety specialist career guide or moving toward clinical leadership in a principal investigator roadmap.
Promotion packet: what to include
Your packet should contain proof, not claims:
A one page impact summary with metrics and outcomes
Two standardized templates you created and implemented
Evidence of training and onboarding support
One example of sponsor communication that reduced risk
A screenshot or log of readiness checks and closed findings
If you want inspiration for organizing evidence, review how structured guides compile resources such as the top 50 remote monitoring tools and model that same clarity in your own portfolio.
The questions you should be ready to answer
Interviewers for Lead CRC roles will test:
How you prevent deviations, not how you report them
How you prioritize when everything is urgent
How you train others and maintain standards
How you handle monitor conflict calmly
How you maintain inspection readiness daily
If you want to strengthen your readiness under pressure, apply structured performance techniques from proven test taking strategies and the disciplined routines described in building a perfect study environment. The mindset is identical: calm, prepared, systematic.
6: FAQs About Becoming a Lead Clinical Research Coordinator
-
It depends less on years and more on proof. Many sites promote within 2 to 4 years if you can run studies with predictable quality, timelines, and communication. The fastest path is to pick one challenging study and build systems that reduce rework, monitor follow ups, and deviations. Use measurable outcomes and artifacts, similar to how structured growth works in a clinical data coordinator career path or a leadership track like the clinical research administrator pathway. If you can show inspection readiness habits and team training impact, you can earn the title faster than time alone would suggest.
-
Build a proof folder. Track 4 metrics for 60 days, publish two standardized workflows, run weekly readiness checks, and document training impact on junior staff. Then package a one page summary that shows results and risk reduction. This is the same evidence based approach that advances careers in sponsor facing paths like the medical science liaison roadmap and compliance focused tracks like the clinical regulatory specialist pathway. If your current site still refuses, your portfolio becomes leverage for external Lead CRC roles.
-
Not always required, but it can accelerate credibility, especially if your site is competitive or you are targeting top institutions. Certifications signal structured knowledge, but promotions still depend on execution and outcomes. Pair learning with proof artifacts such as readiness check logs, standardized workflows, and training plans. If you are preparing for certification exams while working full time, apply the practical focus in proven test taking strategies and optimize your routine using the perfect study environment guide. The best strategy is competence plus evidence, not credentials alone.
-
Common next steps include site manager, clinical trial manager, clinical research administrator, project management roles, or specialized pathways like regulatory or data leadership. Your next step should match what you enjoy: people leadership, compliance, operations, or sponsor facing strategy. If you lean operational, review the clinical research administrator pathway. If you lean data and systems, explore the clinical data manager roadmap. If you lean compliance and submissions, the regulatory affairs specialist roadmap is a strong direction.
-
Common next steps include site manager, clinical trial manager, clinical research administrator, project management roles, or specialized pathways like regulatory or data leadership. Your next step should match what you enjoy: people leadership, compliance, operations, or sponsor facing strategy. If you lean operational, review the clinical research administrator pathway. If you lean data and systems, explore the clinical data manager roadmap. If you lean compliance and submissions, the regulatory affairs specialist roadmap is a strong direction.