Clinical Research Assistant Career Roadmap: Steps and Essential Skills
Clinical research is moving faster than ever, but most trials still break down at the execution layer. That is where Clinical Research Assistants (CRAssts) become the difference between delayed, over-budget studies and clean, inspection-ready data. In this roadmap, you will see exactly how to go from “no experience” to a highly employable Clinical Research Assistant, which skills hiring managers quietly screen for, and how to build a path toward CRA, PV or project manager roles using resources like CCRPS’ clinical regulatory, QA, and project management guides.
1. Why Clinical Research Assistants Are The Backbone Of Modern Trials
Most sponsors worry about principal investigators and funding, but trials usually fail at the operations level. Clinical Research Assistants keep the engine running by coordinating visits, managing documents, and protecting protocol integrity. You are the person who notices when a lab window is missed, when an investigator brochure is outdated, or when a patient is about to drop out.
Strong CRAssts understand how their work plugs into regulatory expectations, using insights from roles like Regulatory Affairs Specialist and Regulatory Affairs Associate. They know that every mis-filed consent form can trigger findings for a future auditor, and they study how clinical quality auditors work by reviewing resources on the clinical quality auditor career pathway.
A modern Clinical Research Assistant also needs a basic understanding of technology and AI trends that shape the tools they use daily. Platforms listed in CCRPS’ top 100 clinical data management and EDC guide, remote monitoring solutions, and patient recruitment companies are already changing day-to-day workflows. High-value CRAssts learn these tools proactively so they are not replaced by the next wave of automation.
The role is also a launchpad. Many senior Clinical Research Associates, pharmacovigilance scientists, and project managers started out as Clinical Research Assistants who deliberately built skills from CCRPS’ pharmacovigilance exam prep resources and CRA certification exam guides.
2. Step By Step Clinical Research Assistant Career Roadmap
Step 1: Build a targeted knowledge base instead of a generic degree.
A life science degree is helpful but not enough. Pair it with focused learning on roles you may grow into, using roadmaps for clinical compliance officers, regulatory specialists and QA specialists. This lets you talk in interviews about how your assistant role will support long term compliance, not just paperwork.
Step 2: Get GCP and structured clinical research training.
Instead of piecing together free videos, follow focused paths like CCRPS’ clinical research associate exam preparation guide or the CRA certification exam question bank. Even if you are not sitting the exam yet, those curricula show you how professional monitors think about protocol deviations, source verification, and subject safety, which immediately upgrades how you perform assistant tasks.
Step 3: Enter the ecosystem in any credible operations role.
Many candidates stall because they wait for the perfect “Clinical Research Assistant” title. Smart candidates accept related positions such as study coordinator intern or data assistant, ideally within organizations listed in CCRPS’ top 50 contract research vendors directory or top 100 pharma and biotech companies hiring PV specialists. Once inside, they volunteer for protocol, regulatory or patient-facing tasks that clearly map to assistant responsibilities.
Step 4: Design your “promotion portfolio” from day one.
Treat your first year as a project. Track metrics like queries closed per month, on-time visit rate, and number of findings resolved before monitoring visits. Document how you improved processes using ideas from articles on AI powered clinical trials, decentralized trials, or AI predicted trial failures. When promotion discussions start, you can prove your value with numbers, not feelings.
Step 5: Add a specialization that aligns with future roles.
If you like safety data, begin working through CCRPS’ pharmacovigilance certification exam questions and PV exam prep guide. If you are drawn to leadership, explore paths toward clinical research project manager certification and project management exam questions. Specialization prevents you from getting stuck as “the person who prints forms” and positions you for either CRA, PV or PM tracks.
Step 6: Prepare for formal exams once experience accumulates.
Within a few years, aim to be exam ready for CRA, PV, or even Principal Investigator certification using CCRPS resources like top 50 PI exam questions and proven PV exam strategies. Your assistant experience will then convert into higher pay, wider responsibility, and more flexible career geography.
3. Essential Technical Skills For Clinical Research Assistants
Protocol literacy and visit mapping.
Clinical Research Assistants who only follow instructions remain replaceable. Those who can interpret protocols, map visit windows, and anticipate operational risks become indispensable. Study how AI-driven tools in predicting patient dropout or remote AI audits flag deviations, and apply similar thinking manually while you work through schedules and logs.
GCP, regulatory and inspection readiness.
Every assistant should know what inspectors look for. Read career guides for clinical compliance officers and clinical quality auditors to understand how missing signatures, outdated IB versions, or unreported SAEs turn into findings. Then design personal checklists that mirror those expectations. This makes you the person who keeps your site inspection ready without being asked.
Data management and EDC fluency.
You do not need to write code, but you must be fluent in data entry logic, edit checks and query patterns across EDC platforms profiled in CCRPS’ top 100 clinical data management and EDC guide. Learn how to interpret query trends to detect protocol misunderstandings early. Over time, you can work with CRAs and data managers to simplify forms, reduce errors, and shorten database lock timelines.
Vendor and tool awareness.
Modern trials run on a matrix of vendors and digital tools. Explore CCRPS directories for remote trial monitoring platforms, patient recruitment firms, and PV training programs. When you understand which vendors support your study, you become the person who can troubleshoot issues and coordinate with external teams without waiting for the project manager.
Technology and AI literacy.
Your future competition is not only other assistants but AI workflows described in CCRPS blogs such as AI powered clinical trials, smart pills and digital biomarkers and virtual reality trials. Learn how these tools collect data, trigger alerts, and integrate into EDC so you can supervise them instead of being replaced by them.
What’s Your Biggest Roadblock To Becoming A Clinical Research Assistant?
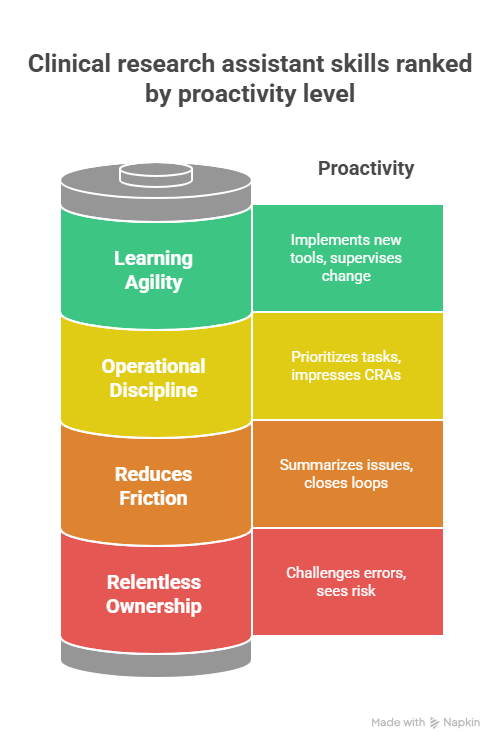
4. High Impact Soft Skills And Daily Habits
Relentless ownership of patient and data safety.
The best Clinical Research Assistants think like compliance officers, not clerks. They challenge missing signatures, unclear AE narratives, and protocol shortcuts even if it means pushing back on busy physicians. Reading pathways for clinical compliance officers, QA specialists, and clinical quality auditors trains your mindset to see risk before others do.
Communication that reduces friction instead of creating it.
Trials fail when coordinators, CRAs, investigators and vendors miscommunicate. Practice writing short, structured emails that summarise the issue, impact and proposed solution, especially when dealing with vendors from lists like CCRPS’ contract research vendors guide or remote PV roles. Over time, people trust you with bigger problems because you consistently close loops.
Operational discipline and prioritisation.
Assistant days are chaotic. Without a system, you will drown in emails and visit windows. Borrow project management ideas from CCRPS’ clinical research project manager exam guide and project management exam questions. Use them to maintain clear daily lists that prioritise safety critical tasks, data entry backlogs and upcoming monitoring visits. This habit is the difference between a site that always looks behind and one that impresses CRAs.
Learning agility in a changing landscape.
Tech giants and AI are entering clinical research, shifting how work is done. Articles on Amazon and Google’s entry into trials, blockchain in trials and AI replacing some clinical jobs can feel threatening. Use them instead as a learning map. Each time you read about a new tool or risk, ask how you can make yourself the person who implements or supervises that change at your site.
5. Future Career Paths For Clinical Research Assistants
Path 1: Clinical Research Associate and remote monitoring roles.
Many assistants move into CRA roles by gradually taking on monitoring-like tasks, then preparing systematically using the CRA certification exam guide, CRA question banks and CRA study resources. Exposure to tools highlighted in the remote monitoring platforms list also prepares you for hybrid or remote CRA positions, which often pay significantly more than assistant roles.
Path 2: Pharmacovigilance and safety science.
If you enjoy AE reporting and safety narratives, pivot into PV roles. Start by mastering content from CCRPS’ pharmacovigilance exam guide, top 50 PV and regulatory exam questions, and proven PV exam strategies. Combine that with market awareness from the top 100 pharma and biotech companies hiring PV specialists and the remote PV jobs list.
Path 3: Regulatory, QA, and compliance leadership.
Assistants who love documents and rules often thrive in regulatory affairs or QA. Resources like the regulatory affairs specialist roadmap, regulatory associate guide, and clinical compliance officer career guide show how to turn that interest into a long term career. You can combine this with experience of inspections and audits gained from working closely with clinical auditors.
Path 4: Principal Investigator and advanced leadership roles.
Some Clinical Research Assistants eventually become coordinators, then sub-investigators and PIs, particularly if they have medical backgrounds. For this path, start early with structured learning from CCRPS’ PI certification exam guide and PI exam question bank. Combine these with innovation focused perspectives from CCRPS’ AI series such as virtual reality clinical trials and augmented reality in trials so you grow into a leader who can design next generation studies, not just traditional ones.
Path 5: Niche roles in tech enabled and decentralized trials.
If you enjoy technology, explore specialized roles around wearables, smart pills and advanced analytics. Articles on smart pills and digital biomarkers, decentralized trials and AI predicted trial failures map out where the industry is moving. Clinical Research Assistants who understand these technologies can transition into roles with tech companies entering clinical research, often with more flexible locations and compensation structures.
6. FAQs: Clinical Research Assistant Career Roadmap And Skills
-
Previous clinical experience helps, but it is not mandatory if you compensate with focused training and smart positioning. Many successful assistants come from laboratory, pharmacy or administrative backgrounds but study structured material from resources like CCRPS’ CRA exam preparation guide and clinical regulatory career paths. Start by understanding GCP, informed consent, and visit workflows, then target entry roles such as clinical data assistant or research coordinator intern where you can prove reliability and attention to detail. Hiring managers mainly want evidence that you understand patient safety, documentation discipline, and can learn quickly inside a regulated environment.
-
In the first few years, your goal is to build broad competence rather than a collection of random certificates. Focus on high quality programs that align with future roles like CRA, PV or project manager. For monitoring oriented careers, use CCRPS’ CRA certification exam guide and CRA exam question bank. If you are drawn to safety, consider resources like the pharmacovigilance exam prep guide and PV training program directory. For leadership tracks, explore project management exam questions and clinical project manager guides.
-
Candidates with clinical licenses often have stronger patient care backgrounds, but you can compete by demonstrating deep operational understanding. Study content on clinical compliance, quality auditing, and regulatory affairs, then use interviews to explain how you will protect protocol integrity and inspection readiness. Prepare examples of how you would handle missing signatures, out of window visits or safety events using principles from CCRPS’ CRA study guide. This type of thinking often impresses hiring managers more than generic clinical experience.
-
Common mistakes include treating the job as pure administration, ignoring metrics, and staying passive when they see risks. New assistants sometimes over focus on data entry and under focus on protocol understanding and patient safety. Many do not track their own impact, so when promotion time comes, they cannot show how they reduced queries or improved visit compliance. Others fail to study how AI and remote tools described in articles like AI powered clinical trials or remote AI audits are changing expectations. Treat your first year as a learning sprint where you document improvements, ask questions, and deliberately build relationships with CRAs, coordinators and data managers.
-
AI and digital tools will automate repetitive work like scheduling reminders, simple queries, and basic data reconciliations, as seen in CCRPS’ series on AI predicted trial failures, patient dropout prediction and AI replacing some jobs. However, these same tools create demand for assistants who can monitor AI outputs, manage decentralized workflows, and integrate data from wearables and smart pills explained in the digital biomarkers guide. If you position yourself as the person who understands both clinical workflows and these technologies, your value will increase rather than disappear.
-
Timelines vary, but with focused effort many assistants reach CRA or junior project manager roles within three to six years. The shortest paths usually combine strong performance metrics, cross training and targeted exam preparation. You might spend the first two years mastering site level operations, then two years taking on more CRA or coordinator responsibilities, while working through the CRA certification exam guide or project manager study resources. Layering in PV or regulatory knowledge from resources like the pharmacovigilance exam guide helps you stand out when leadership roles open, because you can speak across disciplines rather than from a single silo.
-
Yes, especially in environments where assistants gain wide exposure to protocol design, patient interactions and regulatory strategy. If you have or plan to gain medical qualifications, your assistant experience can become a powerful foundation. Pair your daily work with structured learning from CCRPS’ PI certification exam guide, PI exam questions and strategic perspectives from articles on AI driven trials and virtual reality studies. Over time you can progress through coordinator, sub-investigator and PI roles, leading innovative studies with a deep operational understanding that many clinicians lack.